Sunday Poster Session
Category: General Endoscopy
P0679 - Analysis of Reported Adverse Events Related to The Use of The Through-The-Scope Endoscopic Suturing Device: An FDA MAUDE Database Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Abdellatif Ismail, MD
University of Maryland Medical Center
Baltimore, MD
Presenting Author(s)
Abdellatif Ismail, MD1, Ayman Elawad, MD2, Shaikhoon S. Mohammed, MD3, Mohanad Awadalla, MD4, Elmkdad E. Mohammed, MD5, Khalid Aloum, MD6, Hazem Abosheaishaa, MD7, Natalie Wilson, MD8, Tessa Herman, MD9, Monzer Abdalla, MD10, Khalid Ahmed, MD11, Mohamed Abdallah, MD12, Mohammad Bilal, MD9
1University of Maryland Medical Center, Baltimore, MD; 2Massachusetts General Hospital, Boston, MA; 3Emory Clinic/Emory Healthcare, Atlanta, GA; 4Beth Israel Deaconess Medical Center, Boston, MA; 5The Wright Center for Graduate Medical Education, Scranton, PA; 6St. Barnabas Hospital, Bronx, NY; 7Icahn School of Medicine at Mount Sinai, Queens, NY; 8University of Minnesota, Minneapolis, MN; 9University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 10Ascension Saint Francis Hospital, Evanston, IL; 11University of Pennsylvania, Philadelphia, PA; 12Cleveland Clinic, Cleveland, OH
Introduction: The X-Tack Endoscopic HeliX ® Tacking System is a through-the-scope (TTS) suturing device used to close defects in the gastrointestinal tract during endoscopy. The FDA approved the TTS suturing device X-Tack in January 2021 for use in managing fistulas, perforations, and closing defects following endoscopic submucosal dissection or endoscopic mucosal resection. The aim of this study is to analyze adverse events (AE) associated with the X-Tack device using the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database.
Methods: We analyzed the post-marketing surveillance data from the FDA MAUDE database for X-Tack from January 2021 through June 2024. We analyzed patient-related AEs and device-related problems. Additionally, we identified the type of procedure during which these events occurred, (Table 1).
Results: Twenty-three medical device reporting claims were identified. Due to insufficient data in two reports, we analyzed 21 claims. These included patient-related adverse events (n=13, 61.9%) and device-related problems (n=8, 38%). The most common patient-related adverse event was postoperative bleeding (n=5, 38.4%), followed by perforation (n= 2, 15%), abdominal pain (n=2, 15%), pancreatitis (n= 2, 15%), chest pain (n=1, 8%), and nausea/vomiting (n= 1, 8%).
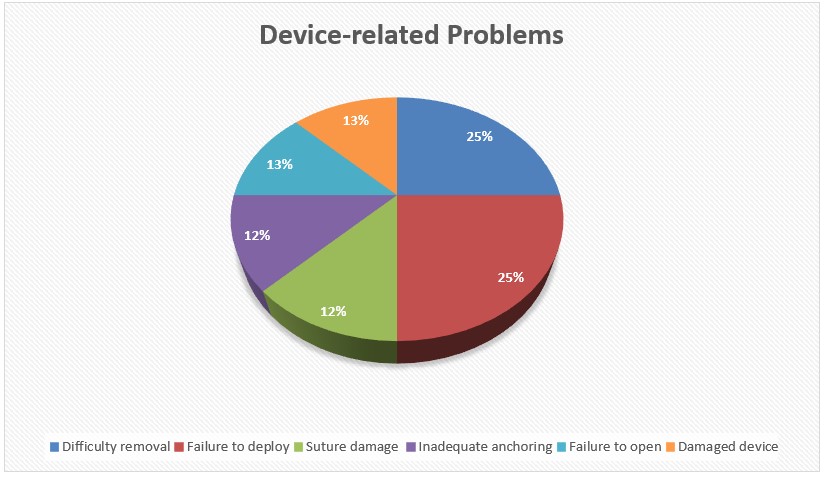
One patient experienced a serious adverse event of bowel perforation following a technically successful mucosal defect closure in the ascending colon. The patient subsequently presented with abdominal pain, and colonic perforation was diagnosed, requiring exploratory laparotomy, right hemicolectomy, and cholecystectomy. In another claim, a patient experienced postoperative bleeding prompting repeat endoscopy for hemostasis. We realize that some of these adverse events may not be related to the use of the device itself. No additional claims provided details on management. The most common device-related problems were failure to deploy (n=2, 25%) and difficulty removal (n=2, 25%), followed by failure to open (n=1, 12.5%), suture damage (n=1, 12.5%), inadequate device anchoring (n=1, 12.5%), and damaged device (n=1, 12.5%), (Figure 1).
Discussion: Our analysis provides endoscopists with valuable information regarding common and rare patient related and device related issues with the X-Tack suturing device. However, due to the inherent nature of the database, there is not enough data to ascertain if these adverse events were secondary to use of the X-Tack suturing device or were related to the underlying clinical pathology.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Abdellatif Ismail, MD1, Ayman Elawad, MD2, Shaikhoon S. Mohammed, MD3, Mohanad Awadalla, MD4, Elmkdad E. Mohammed, MD5, Khalid Aloum, MD6, Hazem Abosheaishaa, MD7, Natalie Wilson, MD8, Tessa Herman, MD9, Monzer Abdalla, MD10, Khalid Ahmed, MD11, Mohamed Abdallah, MD12, Mohammad Bilal, MD9. P0679 - Analysis of Reported Adverse Events Related to The Use of The Through-The-Scope Endoscopic Suturing Device: An FDA MAUDE Database Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Maryland Medical Center, Baltimore, MD; 2Massachusetts General Hospital, Boston, MA; 3Emory Clinic/Emory Healthcare, Atlanta, GA; 4Beth Israel Deaconess Medical Center, Boston, MA; 5The Wright Center for Graduate Medical Education, Scranton, PA; 6St. Barnabas Hospital, Bronx, NY; 7Icahn School of Medicine at Mount Sinai, Queens, NY; 8University of Minnesota, Minneapolis, MN; 9University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN; 10Ascension Saint Francis Hospital, Evanston, IL; 11University of Pennsylvania, Philadelphia, PA; 12Cleveland Clinic, Cleveland, OH
Introduction: The X-Tack Endoscopic HeliX ® Tacking System is a through-the-scope (TTS) suturing device used to close defects in the gastrointestinal tract during endoscopy. The FDA approved the TTS suturing device X-Tack in January 2021 for use in managing fistulas, perforations, and closing defects following endoscopic submucosal dissection or endoscopic mucosal resection. The aim of this study is to analyze adverse events (AE) associated with the X-Tack device using the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database.
Methods: We analyzed the post-marketing surveillance data from the FDA MAUDE database for X-Tack from January 2021 through June 2024. We analyzed patient-related AEs and device-related problems. Additionally, we identified the type of procedure during which these events occurred, (Table 1).
Results: Twenty-three medical device reporting claims were identified. Due to insufficient data in two reports, we analyzed 21 claims. These included patient-related adverse events (n=13, 61.9%) and device-related problems (n=8, 38%). The most common patient-related adverse event was postoperative bleeding (n=5, 38.4%), followed by perforation (n= 2, 15%), abdominal pain (n=2, 15%), pancreatitis (n= 2, 15%), chest pain (n=1, 8%), and nausea/vomiting (n= 1, 8%).
One patient experienced a serious adverse event of bowel perforation following a technically successful mucosal defect closure in the ascending colon. The patient subsequently presented with abdominal pain, and colonic perforation was diagnosed, requiring exploratory laparotomy, right hemicolectomy, and cholecystectomy. In another claim, a patient experienced postoperative bleeding prompting repeat endoscopy for hemostasis. We realize that some of these adverse events may not be related to the use of the device itself. No additional claims provided details on management. The most common device-related problems were failure to deploy (n=2, 25%) and difficulty removal (n=2, 25%), followed by failure to open (n=1, 12.5%), suture damage (n=1, 12.5%), inadequate device anchoring (n=1, 12.5%), and damaged device (n=1, 12.5%), (Figure 1).
Discussion: Our analysis provides endoscopists with valuable information regarding common and rare patient related and device related issues with the X-Tack suturing device. However, due to the inherent nature of the database, there is not enough data to ascertain if these adverse events were secondary to use of the X-Tack suturing device or were related to the underlying clinical pathology.

Figure: Figure 1: Distribution of Device-related problems.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Abdellatif Ismail indicated no relevant financial relationships.
Ayman Elawad indicated no relevant financial relationships.
Shaikhoon Mohammed indicated no relevant financial relationships.
Mohanad Awadalla indicated no relevant financial relationships.
Elmkdad Mohammed indicated no relevant financial relationships.
Khalid Aloum indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Natalie Wilson indicated no relevant financial relationships.
Tessa Herman indicated no relevant financial relationships.
Monzer Abdalla indicated no relevant financial relationships.
Khalid Ahmed indicated no relevant financial relationships.
Mohamed Abdallah indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Abdellatif Ismail, MD1, Ayman Elawad, MD2, Shaikhoon S. Mohammed, MD3, Mohanad Awadalla, MD4, Elmkdad E. Mohammed, MD5, Khalid Aloum, MD6, Hazem Abosheaishaa, MD7, Natalie Wilson, MD8, Tessa Herman, MD9, Monzer Abdalla, MD10, Khalid Ahmed, MD11, Mohamed Abdallah, MD12, Mohammad Bilal, MD9. P0679 - Analysis of Reported Adverse Events Related to The Use of The Through-The-Scope Endoscopic Suturing Device: An FDA MAUDE Database Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
