Sunday Poster Session
Category: General Endoscopy
P0681 - Evaluation of Bowel Preparation Regimens for Colonoscopy in Patients Receiving GLP-1 Receptor Agonists
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Akash Patel, DO
Thomas Jefferson University Hospital
Philadelphia, PA
Presenting Author(s)
Akash Patel, DO1, Beatriz Torre, MD2, Benjamin Young, MD1, Michael J. DiMarino, MD3, Katherine Duffey, MD1, Nicholas Noverati, MD1, Charles Kistler, MD3
1Thomas Jefferson University Hospital, Philadelphia, PA; 2Thomas Jefferson University, Philadelphia, PA; 3Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are being increasingly used for diabetes and weight loss management and while there are many potential benefits, it is well documented that these medications can alter gastrointestinal motility and there is emerging evidence that it can alter the effectiveness of colonoscopy bowel preparation. This study aims to evaluate whether distinctive types of bowel preparation can mitigate the effects of GLP-1RAs on bowel preparation quality.
Methods: We conducted a retrospective chart review of adult patients prescribed GLP-1RAs who underwent outpatient colonoscopies between 2018 and 2024. Data collected included: GLP-1RA regimen, patient demographics, procedure specifics (cecal intubation rates, complications), bowel prep type, and Boston Bowel Prep Score (BBPS). Patients were grouped into two groups based on the bowel preparation they utilized; group 1 included patients who used all prescription preparations and group 2 included patients who used over the counter (OTC) Miralax and Gatorade preparations. T-tests compared mean BBPS, cecal intubation rates, and incompletion rates between the two groups. ANOVA analyzed BBPS differences across various prep types.
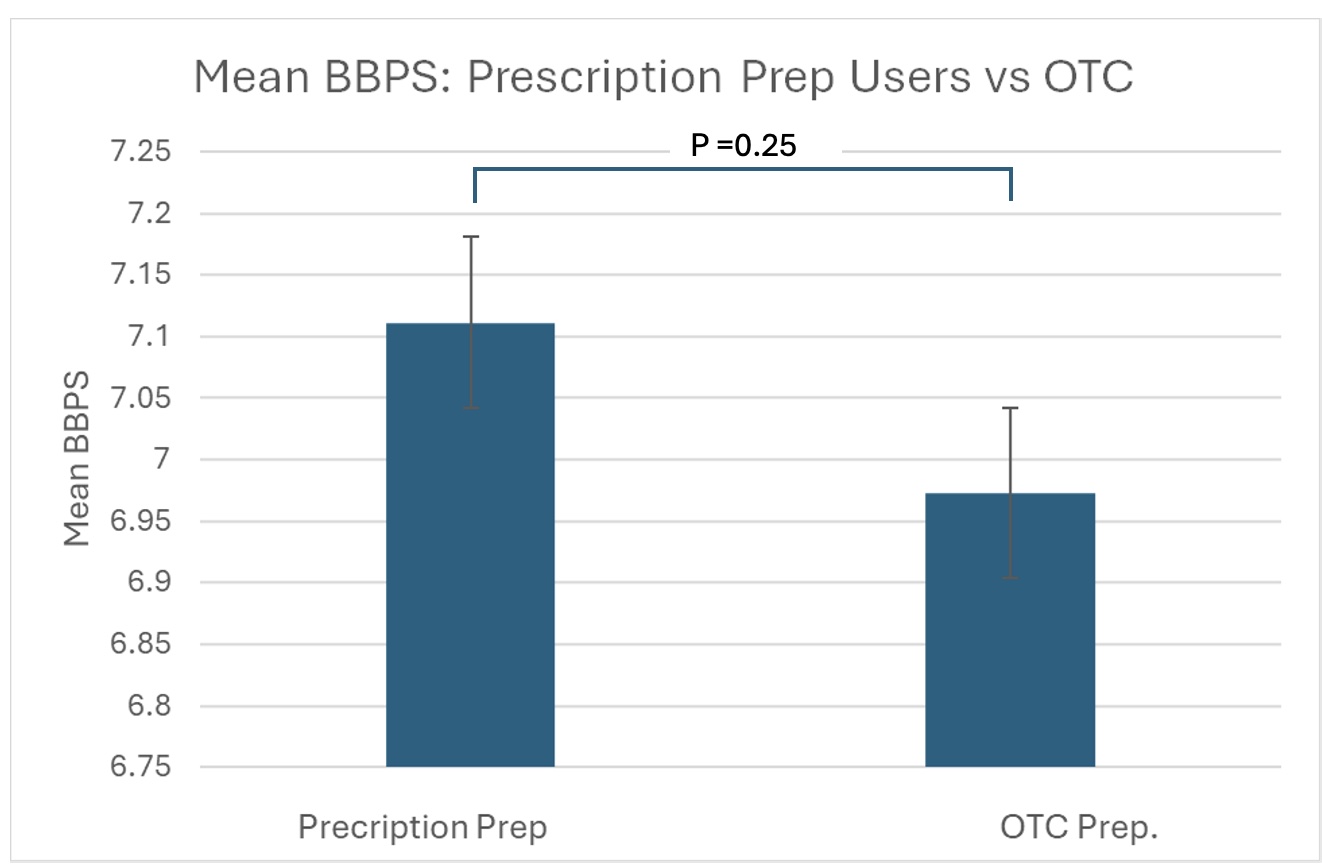
Results: 249 patients were included in our study. Just over half of all patients were white (N=124, 51.8%), and the majority were female (51.8%). The majority of patients were recommended to use a Miralax and Gatorade bowel preparation (182); other prescribed preparations include Colyte (9), Gavilyte (2), GoLytely (26), Moviprep (19), Suprep (9), and Sutab (2). Total mean BBPS was 7.04, with no significant difference between prescribed and OTC groups (7.21 vs 6.97, p = 0.245). Bowel prep inadequacy (BBPS<6) rates didn't significantly differ between groups (8.96% vs 8.79%, p = 0.484). Incomplete procedure rates (1.49% vs. 3.30%, p = 0.223) and cecal intubation rates also did not differ significantly between groups (98.5% vs. 94.51%, p = 0.069).
Discussion: Our study did not show a significant difference in the type of bowel preparation and how it impacts the BBPS in patients taking GLP-1RAs. This may suggest that there is no need for specific bowel prep regimens due to GLP-1RA use alone, although many of these patients may have other risk factors for inadequate preps that warrant such regimens. Further prospective research with larger sample sizes is recommended to explore colonoscopy outcomes in patients receiving GLP-1RAs and various prep regimens.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Akash Patel, DO1, Beatriz Torre, MD2, Benjamin Young, MD1, Michael J. DiMarino, MD3, Katherine Duffey, MD1, Nicholas Noverati, MD1, Charles Kistler, MD3. P0681 - Evaluation of Bowel Preparation Regimens for Colonoscopy in Patients Receiving GLP-1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Thomas Jefferson University Hospital, Philadelphia, PA; 2Thomas Jefferson University, Philadelphia, PA; 3Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are being increasingly used for diabetes and weight loss management and while there are many potential benefits, it is well documented that these medications can alter gastrointestinal motility and there is emerging evidence that it can alter the effectiveness of colonoscopy bowel preparation. This study aims to evaluate whether distinctive types of bowel preparation can mitigate the effects of GLP-1RAs on bowel preparation quality.
Methods: We conducted a retrospective chart review of adult patients prescribed GLP-1RAs who underwent outpatient colonoscopies between 2018 and 2024. Data collected included: GLP-1RA regimen, patient demographics, procedure specifics (cecal intubation rates, complications), bowel prep type, and Boston Bowel Prep Score (BBPS). Patients were grouped into two groups based on the bowel preparation they utilized; group 1 included patients who used all prescription preparations and group 2 included patients who used over the counter (OTC) Miralax and Gatorade preparations. T-tests compared mean BBPS, cecal intubation rates, and incompletion rates between the two groups. ANOVA analyzed BBPS differences across various prep types.
Results: 249 patients were included in our study. Just over half of all patients were white (N=124, 51.8%), and the majority were female (51.8%). The majority of patients were recommended to use a Miralax and Gatorade bowel preparation (182); other prescribed preparations include Colyte (9), Gavilyte (2), GoLytely (26), Moviprep (19), Suprep (9), and Sutab (2). Total mean BBPS was 7.04, with no significant difference between prescribed and OTC groups (7.21 vs 6.97, p = 0.245). Bowel prep inadequacy (BBPS<6) rates didn't significantly differ between groups (8.96% vs 8.79%, p = 0.484). Incomplete procedure rates (1.49% vs. 3.30%, p = 0.223) and cecal intubation rates also did not differ significantly between groups (98.5% vs. 94.51%, p = 0.069).
Discussion: Our study did not show a significant difference in the type of bowel preparation and how it impacts the BBPS in patients taking GLP-1RAs. This may suggest that there is no need for specific bowel prep regimens due to GLP-1RA use alone, although many of these patients may have other risk factors for inadequate preps that warrant such regimens. Further prospective research with larger sample sizes is recommended to explore colonoscopy outcomes in patients receiving GLP-1RAs and various prep regimens.

Figure: Mean BBPS in prescription versus OTC preparation groups
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Akash Patel indicated no relevant financial relationships.
Beatriz Torre indicated no relevant financial relationships.
Benjamin Young indicated no relevant financial relationships.
Michael DiMarino indicated no relevant financial relationships.
Katherine Duffey indicated no relevant financial relationships.
Nicholas Noverati indicated no relevant financial relationships.
Charles Kistler indicated no relevant financial relationships.
Akash Patel, DO1, Beatriz Torre, MD2, Benjamin Young, MD1, Michael J. DiMarino, MD3, Katherine Duffey, MD1, Nicholas Noverati, MD1, Charles Kistler, MD3. P0681 - Evaluation of Bowel Preparation Regimens for Colonoscopy in Patients Receiving GLP-1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
