Sunday Poster Session
Category: GI Bleeding
P0730 - Comparison of TC-325 Hemostatic Powder With Standard Endoscopic Treatments for Malignancy-Related Upper Gastrointestinal GI Bleeding: Meta-Analysis of Randomized Controlled Trials
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio
- FK
Faisal Kamal, MD
Sidney Kimmel Medical College at Thomas Jefferson University
Philadelphia, PA
Presenting Author(s)
Award: Presidential Poster Award
Aamir Saeed, MD1, Saira Yousuf, MD1, Muhammad Hayat, MD1, Marjan Haider, MD2, Manesh Kumar Gangwani, MD3, Muhammad Aziz, MD4, Umar Hayat, MD5, Christian Salcedo, MD6, Umer Farooq, MD7, Nasir Saleem, MD8, Sachit Sharma, MD9, Muhammad Khan, MD10, Faisal Kamal, MD11
1Vanderbilt University Medical Center, Nashville, TN; 2Trinity Health Ann Arbor Hospital, Ann Arbor, MI; 3University of Toledo, Toledo, OH; 4Bon Secours Mercy, Toledo, OH; 5Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA; 6University of Tennessee Health Science Center, Memphis, TN; 7SSM Health Saint Louis University Hospital, St. Louis, MO; 8Indiana University School of Medicine, Indianapolis, IN; 9Virginia Commonwealth University Medical Center, Richmond, VA; 10MD Anderson Cancer Center, Houston, TX; 11Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA
Introduction: Management of malignancy-related upper gastrointestinal bleeding (UGIB) is challenging and conventional endoscopic treatments have variable rates of success in achieving hemostasis and risk of rebleeding and mortality is high. Lately, TC-325 powder (Hemospray; Cook Medical, Winston-Salem, North Carolina, United States) has been successfully used in the management of malignancy related UGIB. We conducted a meta-analysis of randomized controlled trials (RCTs) comparing TC-325 hemostatic powder with standard endoscopic treatments in the management of malignancy related UGIB.
Methods: Several databases were reviewed from inception to May 02, 2024 to identify RCTs comparing TC-325 and standard endoscopic treatments for management of malignancy-related UGIB. Our outcomes of interest were immediate hemostasis, 30-day rebleeding, length of hospital stay, need for surgery, need for angiographic embolization and all-cause mortality. We calculated pooled risk ratio (RR) with 95% confidence intervals (CI) for categorical variables and mean difference (MD) with 95% CI for continuous variables. We used a random effect model to analyze the data. We assessed heterogeneity using the I2 statistic.
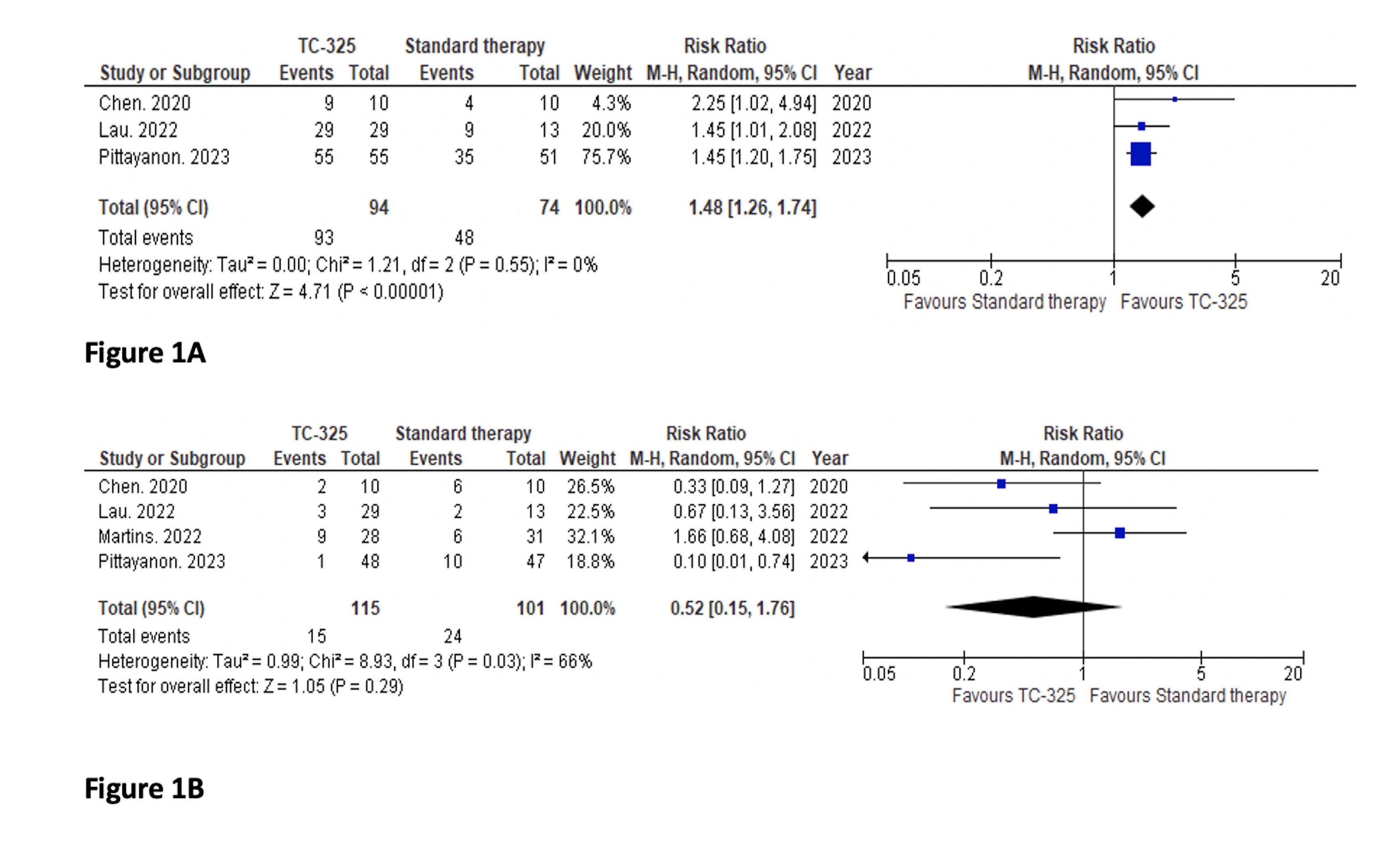
Results: Four RCTs with 227 patients (122 patients in TC-325 group and 105 patients in standard treatment group) were included in the final analysis. We found that, the rate of immediate hemostasis was significantly higher in TC-325 group compared to the standard therapy group, RR (95% CI): 1.48 (1.26, 1.74), I² = 0% (Figure 1A). There was no significant difference in 30-day rebleeding between the groups RR (95% CI): 0.52 (0.15, 1.76), I² = 66% (Figure 1B). We found no significant difference in other outcomes between groups such as need for angiographic embolization; RR (95% CI): 1.07 (0.18, 6.22), I² = 12%, all cause mortality; RR (95% CI): 1.12 (0.77, 1.63), I²=0%, length of hospital stay; MD (95% CI): 4.28 (-0.27, 8.82), I² = 0% and need for surgery; RR (95% CI): 0.65 (0.37, 1.14), I² =0%.
Discussion: Our meta-analysis demonstrates the superiority of TC-325 hemostatic powder over standard endoscopic treatments in patients with malignancy related UGIB in achieving better immediate hemostasis. We found no significant difference in other outcomes between groups. Due to ease of its application, TC-325 powder can be considered as the first line treatment for patients with UGIB related to malignancies.
Disclosures:
Aamir Saeed, MD1, Saira Yousuf, MD1, Muhammad Hayat, MD1, Marjan Haider, MD2, Manesh Kumar Gangwani, MD3, Muhammad Aziz, MD4, Umar Hayat, MD5, Christian Salcedo, MD6, Umer Farooq, MD7, Nasir Saleem, MD8, Sachit Sharma, MD9, Muhammad Khan, MD10, Faisal Kamal, MD11. P0730 - Comparison of TC-325 Hemostatic Powder With Standard Endoscopic Treatments for Malignancy-Related Upper Gastrointestinal GI Bleeding: Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Aamir Saeed, MD1, Saira Yousuf, MD1, Muhammad Hayat, MD1, Marjan Haider, MD2, Manesh Kumar Gangwani, MD3, Muhammad Aziz, MD4, Umar Hayat, MD5, Christian Salcedo, MD6, Umer Farooq, MD7, Nasir Saleem, MD8, Sachit Sharma, MD9, Muhammad Khan, MD10, Faisal Kamal, MD11
1Vanderbilt University Medical Center, Nashville, TN; 2Trinity Health Ann Arbor Hospital, Ann Arbor, MI; 3University of Toledo, Toledo, OH; 4Bon Secours Mercy, Toledo, OH; 5Geisinger Wyoming Valley Medical Center, Wilkes-Barre, PA; 6University of Tennessee Health Science Center, Memphis, TN; 7SSM Health Saint Louis University Hospital, St. Louis, MO; 8Indiana University School of Medicine, Indianapolis, IN; 9Virginia Commonwealth University Medical Center, Richmond, VA; 10MD Anderson Cancer Center, Houston, TX; 11Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA
Introduction: Management of malignancy-related upper gastrointestinal bleeding (UGIB) is challenging and conventional endoscopic treatments have variable rates of success in achieving hemostasis and risk of rebleeding and mortality is high. Lately, TC-325 powder (Hemospray; Cook Medical, Winston-Salem, North Carolina, United States) has been successfully used in the management of malignancy related UGIB. We conducted a meta-analysis of randomized controlled trials (RCTs) comparing TC-325 hemostatic powder with standard endoscopic treatments in the management of malignancy related UGIB.
Methods: Several databases were reviewed from inception to May 02, 2024 to identify RCTs comparing TC-325 and standard endoscopic treatments for management of malignancy-related UGIB. Our outcomes of interest were immediate hemostasis, 30-day rebleeding, length of hospital stay, need for surgery, need for angiographic embolization and all-cause mortality. We calculated pooled risk ratio (RR) with 95% confidence intervals (CI) for categorical variables and mean difference (MD) with 95% CI for continuous variables. We used a random effect model to analyze the data. We assessed heterogeneity using the I2 statistic.
Results: Four RCTs with 227 patients (122 patients in TC-325 group and 105 patients in standard treatment group) were included in the final analysis. We found that, the rate of immediate hemostasis was significantly higher in TC-325 group compared to the standard therapy group, RR (95% CI): 1.48 (1.26, 1.74), I² = 0% (Figure 1A). There was no significant difference in 30-day rebleeding between the groups RR (95% CI): 0.52 (0.15, 1.76), I² = 66% (Figure 1B). We found no significant difference in other outcomes between groups such as need for angiographic embolization; RR (95% CI): 1.07 (0.18, 6.22), I² = 12%, all cause mortality; RR (95% CI): 1.12 (0.77, 1.63), I²=0%, length of hospital stay; MD (95% CI): 4.28 (-0.27, 8.82), I² = 0% and need for surgery; RR (95% CI): 0.65 (0.37, 1.14), I² =0%.
Discussion: Our meta-analysis demonstrates the superiority of TC-325 hemostatic powder over standard endoscopic treatments in patients with malignancy related UGIB in achieving better immediate hemostasis. We found no significant difference in other outcomes between groups. Due to ease of its application, TC-325 powder can be considered as the first line treatment for patients with UGIB related to malignancies.

Figure: Comparison of immediate hemostasis (Figure 1A) and 30day rebleeding (Figure 1B) between groups
Disclosures:
Aamir Saeed indicated no relevant financial relationships.
Saira Yousuf indicated no relevant financial relationships.
Muhammad Hayat indicated no relevant financial relationships.
Marjan Haider indicated no relevant financial relationships.
Manesh Kumar Gangwani indicated no relevant financial relationships.
Muhammad Aziz indicated no relevant financial relationships.
Umar Hayat indicated no relevant financial relationships.
Christian Salcedo indicated no relevant financial relationships.
Umer Farooq indicated no relevant financial relationships.
Nasir Saleem indicated no relevant financial relationships.
Sachit Sharma indicated no relevant financial relationships.
Muhammad Khan indicated no relevant financial relationships.
Faisal Kamal indicated no relevant financial relationships.
Aamir Saeed, MD1, Saira Yousuf, MD1, Muhammad Hayat, MD1, Marjan Haider, MD2, Manesh Kumar Gangwani, MD3, Muhammad Aziz, MD4, Umar Hayat, MD5, Christian Salcedo, MD6, Umer Farooq, MD7, Nasir Saleem, MD8, Sachit Sharma, MD9, Muhammad Khan, MD10, Faisal Kamal, MD11. P0730 - Comparison of TC-325 Hemostatic Powder With Standard Endoscopic Treatments for Malignancy-Related Upper Gastrointestinal GI Bleeding: Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

