Sunday Poster Session
Category: IBD
P0826 - Efficacy and Safety of Guselkumab Maintenance Therapy Among Guselkumab Induction Week 24 Clinical Responders: Results From the Phase 3 QUASAR Maintenance Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
- DR
David T. Rubin, MD, FACG
University of Chicago Medicine, Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
Award: Presidential Poster Award
David T. Rubin, MD, FACG1, Axel Dignass, MD, PhD2, Jessica R. Allegretti, MD, MPH, FACG3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Jia Zhan, PhD5, Hongyan Zhang, PhD5, Yaser Rayyan, MD6, Masayuki Saruta, MD, PhD7, Domingo Balderramo, MD, PhD8, Brian Bressler, MS, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 2Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 3Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Research and Development, Spring House, PA; 6The University of Jordan, School of Medicine, Amman, 'Amman, Jordan; 7The Jikei University School of Medicine, Tokyo, Tokyo, Japan; 8Hospital Privado Universitario de Córdoba, Instituto Universitario de Ciencias Biomédicas, Córdoba, Cordoba, Argentina; 9University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada; 10INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 11Kyorin University School of Medicine, Tokyo, Tokyo, Japan
Introduction: The QUASAR program (NCT04033445) evaluated guselkumab (GUS), a dual-acting interleukin-23p19 subunit inhibitor, in patients (pts) with moderately to severely active ulcerative colitis (UC). Induction Week 12 (Week I-12) nonresponders to intravenous (IV) GUS received subcutaneous (SC) GUS through Week I-24.1 Week I‑24 responders were eligible for maintenance therapy. Here, we present the efficacy and safety of maintenance SC GUS among GUS Week I-24 responders.
Methods: Pts who were not in clinical response (definition in Table) at Week I-12 after IV GUS 200 mg or 400 mg received SC GUS 200 mg at Weeks I-12, I-16, and I-20. Those who were in clinical response at Week I-24 received SC GUS 200 mg q4w during the maintenance study in a blinded fashion and were evaluated as part of the nonrandomized study population. Clinical, symptomatic, endoscopic, histologic, and patient-reported outcome measures at maintenance Week 44 (Week M-44) and safety throughout the maintenance study are reported for GUS Week I-24 responders.
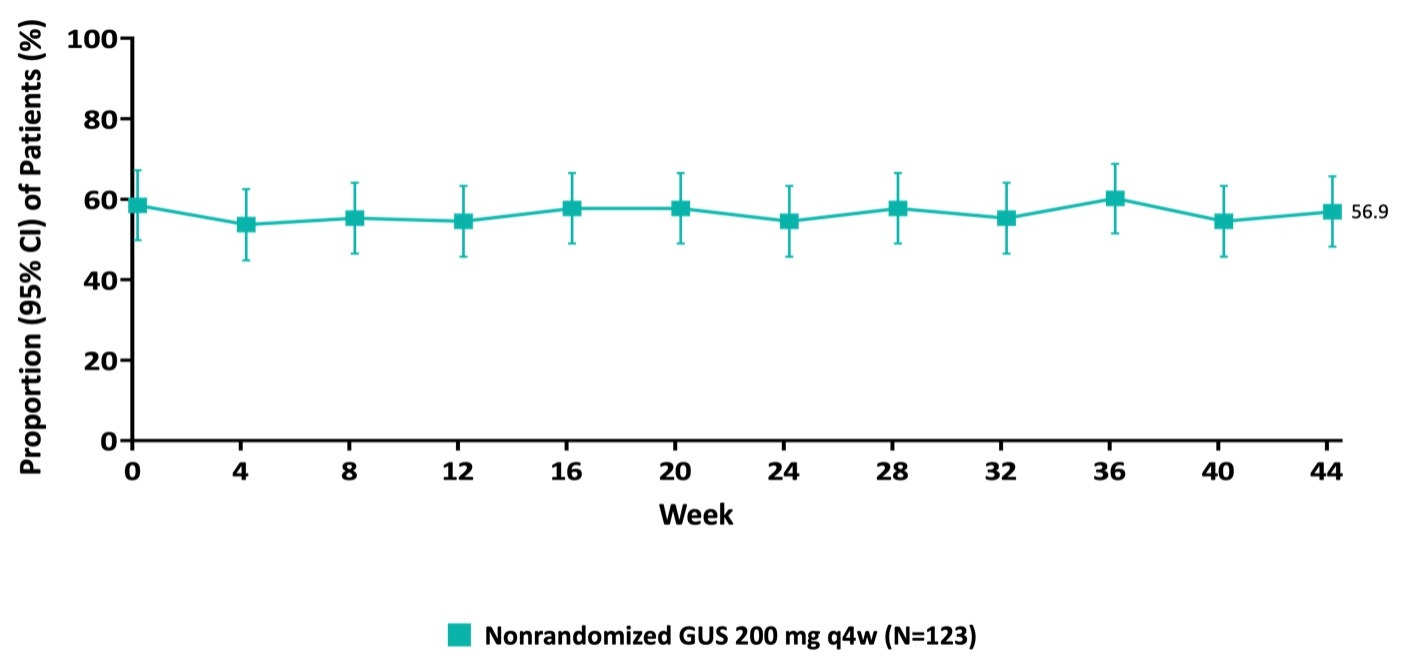
Results: Overall, 123 of 203 (60.6%) Week I-12 nonresponders to IV GUS achieved clinical response at Week I-24 and entered the maintenance study phase. Induction baseline characteristics of GUS Week I-24 responders were: 74.8% had severe disease (modified Mayo score 7-9), 77.2% had a Mayo endoscopy subscore of 3, median CRP was 5.0 mg/L (upper limit of normal, 3 mg/L), and 59.3% had a history of documented inadequate response or intolerance to biologic, or JAK inhibitor therapy for UC. At Week M‑44, 67.5% of the pts maintained clinical response and 30.1% were in clinical remission. Additional efficacy outcomes are shown in the Table. The proportion of GUS Week I-24 responders in symptomatic remission (defined as a stool frequency subscore of 0 or 1 and not increased from induction baseline, and a rectal bleeding subscore of 0) at maintenance baseline (58.5%) was sustained through Week M‑44 (56.9%) (Figure). Adverse events (AEs) were reported for 78.0% of GUS Week I-24 responders, serious AEs for 5.7%, and serious infections for 1.6%. No opportunistic infections or deaths occurred. No new safety concerns were identified.
Discussion: In this refractory population of GUS Week I-24 responders, maintenance treatment with GUS provided clinical benefit across a range of clinically relevant efficacy endpoints. Safety results were consistent with the overall population and safety profile of GUS in its approved indications.
1. Bressler B, et al. Am J Gastroenterol. 2023;118(10S):806-807.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
David T. Rubin, MD, FACG1, Axel Dignass, MD, PhD2, Jessica R. Allegretti, MD, MPH, FACG3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Jia Zhan, PhD5, Hongyan Zhang, PhD5, Yaser Rayyan, MD6, Masayuki Saruta, MD, PhD7, Domingo Balderramo, MD, PhD8, Brian Bressler, MS, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11. P0826 - Efficacy and Safety of Guselkumab Maintenance Therapy Among Guselkumab Induction Week 24 Clinical Responders: Results From the Phase 3 QUASAR Maintenance Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
David T. Rubin, MD, FACG1, Axel Dignass, MD, PhD2, Jessica R. Allegretti, MD, MPH, FACG3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Jia Zhan, PhD5, Hongyan Zhang, PhD5, Yaser Rayyan, MD6, Masayuki Saruta, MD, PhD7, Domingo Balderramo, MD, PhD8, Brian Bressler, MS, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11
1University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 2Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 3Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Research and Development, Spring House, PA; 6The University of Jordan, School of Medicine, Amman, 'Amman, Jordan; 7The Jikei University School of Medicine, Tokyo, Tokyo, Japan; 8Hospital Privado Universitario de Córdoba, Instituto Universitario de Ciencias Biomédicas, Córdoba, Cordoba, Argentina; 9University of British Columbia, IBD Centre of BC, Vancouver, BC, Canada; 10INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 11Kyorin University School of Medicine, Tokyo, Tokyo, Japan
Introduction: The QUASAR program (NCT04033445) evaluated guselkumab (GUS), a dual-acting interleukin-23p19 subunit inhibitor, in patients (pts) with moderately to severely active ulcerative colitis (UC). Induction Week 12 (Week I-12) nonresponders to intravenous (IV) GUS received subcutaneous (SC) GUS through Week I-24.1 Week I‑24 responders were eligible for maintenance therapy. Here, we present the efficacy and safety of maintenance SC GUS among GUS Week I-24 responders.
Methods: Pts who were not in clinical response (definition in Table) at Week I-12 after IV GUS 200 mg or 400 mg received SC GUS 200 mg at Weeks I-12, I-16, and I-20. Those who were in clinical response at Week I-24 received SC GUS 200 mg q4w during the maintenance study in a blinded fashion and were evaluated as part of the nonrandomized study population. Clinical, symptomatic, endoscopic, histologic, and patient-reported outcome measures at maintenance Week 44 (Week M-44) and safety throughout the maintenance study are reported for GUS Week I-24 responders.
Results: Overall, 123 of 203 (60.6%) Week I-12 nonresponders to IV GUS achieved clinical response at Week I-24 and entered the maintenance study phase. Induction baseline characteristics of GUS Week I-24 responders were: 74.8% had severe disease (modified Mayo score 7-9), 77.2% had a Mayo endoscopy subscore of 3, median CRP was 5.0 mg/L (upper limit of normal, 3 mg/L), and 59.3% had a history of documented inadequate response or intolerance to biologic, or JAK inhibitor therapy for UC. At Week M‑44, 67.5% of the pts maintained clinical response and 30.1% were in clinical remission. Additional efficacy outcomes are shown in the Table. The proportion of GUS Week I-24 responders in symptomatic remission (defined as a stool frequency subscore of 0 or 1 and not increased from induction baseline, and a rectal bleeding subscore of 0) at maintenance baseline (58.5%) was sustained through Week M‑44 (56.9%) (Figure). Adverse events (AEs) were reported for 78.0% of GUS Week I-24 responders, serious AEs for 5.7%, and serious infections for 1.6%. No opportunistic infections or deaths occurred. No new safety concerns were identified.
Discussion: In this refractory population of GUS Week I-24 responders, maintenance treatment with GUS provided clinical benefit across a range of clinically relevant efficacy endpoints. Safety results were consistent with the overall population and safety profile of GUS in its approved indications.
1. Bressler B, et al. Am J Gastroenterol. 2023;118(10S):806-807.

Figure: Figure. Symptomatic Remission (a) Through Maintenance Week 44 for GUS Induction Week 24 Clinical Responders.
CI-confidence interval; GUS=guselkumab; q4w=every 4 weeks.
a Symptomatic remission was defined as a stool frequency subscore of 0 or 1 and not increased from induction baseline, and a rectal bleeding subscore of 0.
CI-confidence interval; GUS=guselkumab; q4w=every 4 weeks.
a Symptomatic remission was defined as a stool frequency subscore of 0 or 1 and not increased from induction baseline, and a rectal bleeding subscore of 0.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
David T. Rubin: AbbVie – Consultant. Altrubio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol-Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. Connect BioPharma – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Axel Dignass: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Abivax – Advisor or Review Panel Member, Advisory Committee/Board Member. Amgen – Consultant. Arena Pharmaceuticals – Consultant. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Speakers Bureau. High5MD – Speakers Bureau. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Payment for manuscript preparation, Speakers Bureau. Lilly – Consultant. Materia Prima – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Consultant. Roche – Consultant. Sandoz – Consultant, Speakers Bureau. Stada – Consultant. Takeda – Consultant, Payment for manuscript preparation, Speakers Bureau. Thieme – Payment for manuscript preparation. Tillotts – Consultant, Speakers Bureau. UniMed Verlag – Payment for manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Jessica R. Allegretti: Abbvie – Consultant, Speakers Bureau. Artugen – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant. Finch Therapeutics – Consultant. Janssen – Consultant. Merck – Grant/Research Support. Pfizer – Consultant. Seres – Consultant.
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jia Zhan: Johnson & Johnson – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Yaser Rayyan indicated no relevant financial relationships.
Masayuki Saruta: AbbVie GK – payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events. EA Pharma – Grant/Research Support, honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events, Speakers Bureau. EPS Corporation – Grant/Research Support. Gilead Sciences – payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events. Janssen Pharmaceutical – payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events. Kissei Pharmaceutical – Grant/Research Support, payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events, Speakers Bureau. Mitsubishi Tanabe Pharma – payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events. Mochida Pharmaceutical – Grant/Research Support. Takeda Pharmaceutical – payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events. Zeria Pharma – Grant/Research Support.
Domingo Balderramo: AbbVie – Advisor or Review Panel Member, travel/congress support, Speakers Bureau. Amgen – Advisor or Review Panel Member, Speakers Bureau. Ferring – travel/congress support. Janssen – Advisor or Review Panel Member, travel/congress support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Takeda – Advisor or Review Panel Member, travel/congress support, Speakers Bureau.
Brian Bressler: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Amgen – Advisory Committee/Board Member, Grant/Research Support. AMT – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Grant/Research Support. Celgene – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Eupraxia – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Genentech/Roche – Advisory Committee/Board Member, Grant/Research Support. Gilead – Advisory Committee/Board Member. GSK – Grant/Research Support. Iterative Scopes – Advisory Committee/Board Member. Jamp – Advisor or Review Panel Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Merck – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisor or Review Panel Member. Mylan – Advisor or Review Panel Member. Novartis – Advisory Committee/Board Member, Speakers Bureau. Organon – Advisory Committee/Board Member, Speakers Bureau. Pendopharm – Advisor or Review Panel Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Protagonist – Advisor or Review Panel Member. Qu Biologic – Grant/Research Support, Stock Options. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Takeda – Advisory Committee/Board Member, Speakers Bureau. Viatris – Advisor or Review Panel Member.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Tadakazu Hisamatsu: AbbVie – Grant/Research Support, lecture fees. Bristol Myers Squibb – Consultant. Daiichi-Sankyo – Grant/Research Support. EA Pharma – Consultant, Grant/Research Support, lecture fees. Gilead Sciences – Consultant. Janssen – Consultant. JIMRO – Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, lecture fees. Mochida Pharmaceutical – Grant/Research Support. Nippon Kayaku – Grant/Research Support. Pfizer – Grant/Research Support. Takeda Pharmaceutical – Grant/Research Support, lecture fees.
David T. Rubin, MD, FACG1, Axel Dignass, MD, PhD2, Jessica R. Allegretti, MD, MPH, FACG3, Shadi Yarandi, PhD4, Kuan-Hsiang G. Huang, MD, PhD5, Matthew Germinaro, MD5, Jia Zhan, PhD5, Hongyan Zhang, PhD5, Yaser Rayyan, MD6, Masayuki Saruta, MD, PhD7, Domingo Balderramo, MD, PhD8, Brian Bressler, MS, MD9, Laurent Peyrin-Biroulet, MD, PhD10, Tadakazu Hisamatsu, MD, PhD11. P0826 - Efficacy and Safety of Guselkumab Maintenance Therapy Among Guselkumab Induction Week 24 Clinical Responders: Results From the Phase 3 QUASAR Maintenance Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

