Sunday Poster Session
Category: IBD
P0849 - Corticosteroid-Free Remission Through 3 Years of Upadacitinib Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Data From the Phase 3 Long-Term Extension Study U-ACTIVATE
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Marla Dubinsky, MD
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Marla Dubinsky, MD1, Raja Atreya, MD2, Hiroshi Nakase, MD3, Michelle Kujawski, PhD4, James Crooks, PhD5, Erica Cheng, PhD4, Justin Klaff, MD4, Remo Panaccione, MD6
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Erlangen University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Baden-Wurttemberg, Germany; 3Sapporo Medical University School of Medicine, Sapporo, Hokkaido, Japan; 4AbbVie Inc., North Chicago, IL; 5AbbVie Inc., Maidenhead, England, United Kingdom; 6University of Calgary, Calgary, AB, Canada
Introduction: Upadacitinib (UPA), an oral, selective, reversible Janus kinase (JAK) inhibitor, demonstrated efficacy vs placebo (PBO) across clinical, endoscopic, and histologic endpoints, was efficacious as a corticosteroid (CS)-sparing treatment, and was well tolerated in the U-ACHIEVE maintenance study.1-3 Here, we report the achievement of CS-free clinical remission (CR) through 96 weeks in the ongoing, 288-week, U-ACTIVATE long-term extension (LTE) in patients with moderately to severely active ulcerative colitis (UC).
Methods: Data from clinical responders to 8 weeks of induction,1,4 who completed 52 weeks of maintenance (UPA 15 mg [UPA15] once daily [QD] or UPA 30 mg [UPA30] QD) and entered the LTE, were analyzed. CS-free CR (Adapted Mayo score ≤ 2, with stool frequency subscore ≤ 1 and not greater than baseline, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1) was evaluated at LTE week 96 in clinical responders (per Adapted Mayo score) at maintenance week 0, without CS use at LTE week 96, that stayed on the same dose in the LTE (UPA15/15 and UPA30/30 groups). Data are presented as observed.
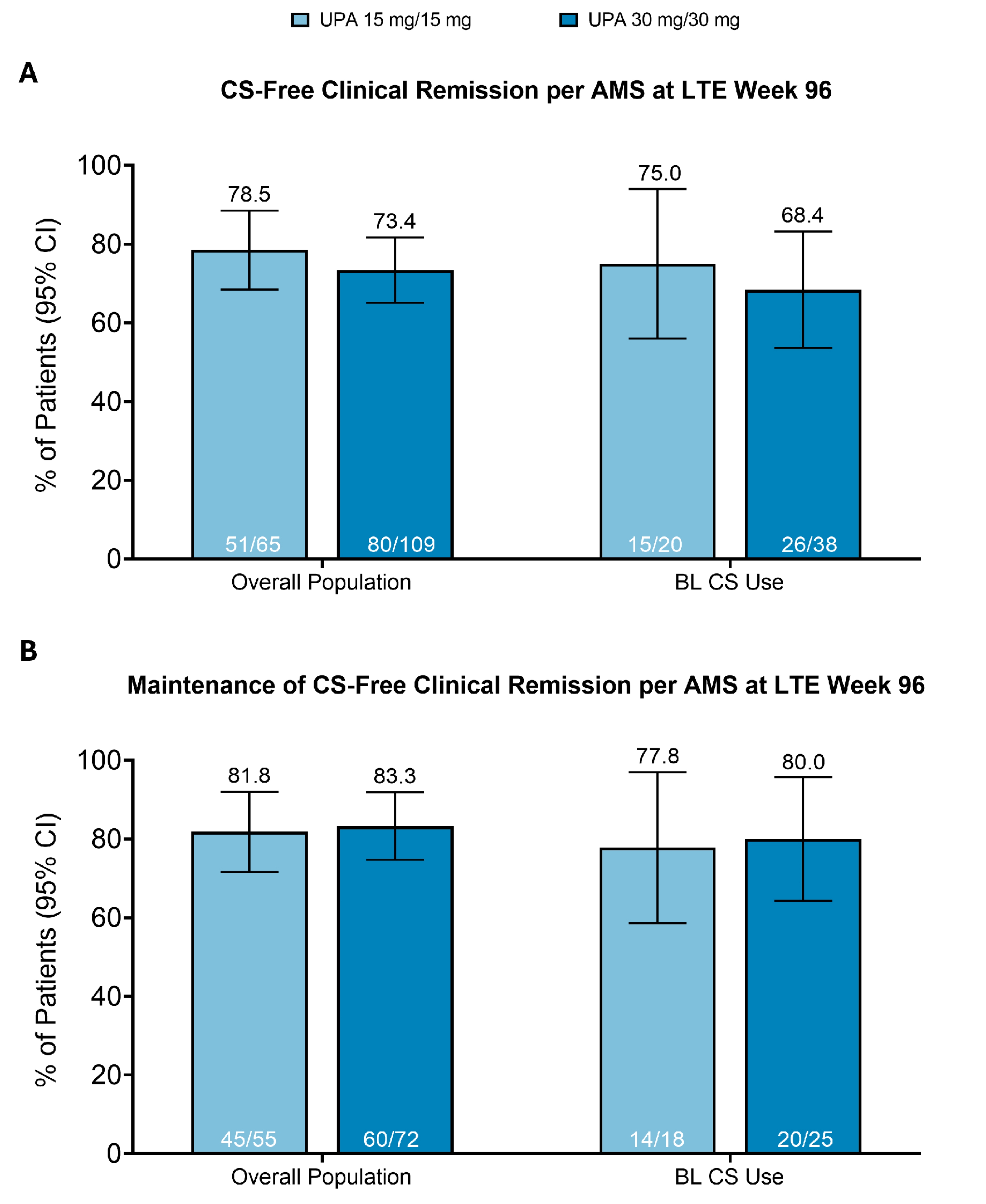
Results: Among patients in the overall population who entered the LTE, 78.5% (n=51/65) and 73.4% (80/109) in the UPA15/15 and UPA30/30 groups, respectively, achieved CS-free CR at LTE week 96 (Figure 1A). Additionally, among patients with CS use at induction baseline who entered the LTE, 75.0% (15/20) and 68.4% (26/38) in the UPA15/15 and UPA30/30 groups, respectively, achieved CS-free CR at LTE week 96. Maintenance of CS-free CR at LTE week 96 was achieved by 81.8% (45/55) and 83.3% (60/72) of patients in the UPA15/15 and UPA30/30 groups, respectively, in the overall population (Figure 1B). Additionally, maintenance of CS-free CR was achieved by 77.8% (14/18) and 80.0% (20/25) of patients in the UPA15/15 and UPA30/30 groups, respectively, with CS use at induction baseline.
Discussion: Although the number of patients analyzed was limited, most patients achieved and maintained CS-free CR through LTE week 96. These findings suggest the benefit of UPA as a long-term, CS-sparing treatment option for moderately to severely active UC.
References:
1. Danese S et al. Lancet 2022;399:2113–28
2. Raine T et al. J Crohn’s Colitis 2024;18(5):695–707
3. Colombel J-F et al. J Crohn's and Colitis. 2022;16(S1):i514
4. Vermeire S et al. Lancet Gastroenterol Hepatol 2023;8:976–89
Disclosures:
Marla Dubinsky, MD1, Raja Atreya, MD2, Hiroshi Nakase, MD3, Michelle Kujawski, PhD4, James Crooks, PhD5, Erica Cheng, PhD4, Justin Klaff, MD4, Remo Panaccione, MD6. P0849 - Corticosteroid-Free Remission Through 3 Years of Upadacitinib Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Data From the Phase 3 Long-Term Extension Study U-ACTIVATE, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Erlangen University Hospital, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Baden-Wurttemberg, Germany; 3Sapporo Medical University School of Medicine, Sapporo, Hokkaido, Japan; 4AbbVie Inc., North Chicago, IL; 5AbbVie Inc., Maidenhead, England, United Kingdom; 6University of Calgary, Calgary, AB, Canada
Introduction: Upadacitinib (UPA), an oral, selective, reversible Janus kinase (JAK) inhibitor, demonstrated efficacy vs placebo (PBO) across clinical, endoscopic, and histologic endpoints, was efficacious as a corticosteroid (CS)-sparing treatment, and was well tolerated in the U-ACHIEVE maintenance study.1-3 Here, we report the achievement of CS-free clinical remission (CR) through 96 weeks in the ongoing, 288-week, U-ACTIVATE long-term extension (LTE) in patients with moderately to severely active ulcerative colitis (UC).
Methods: Data from clinical responders to 8 weeks of induction,1,4 who completed 52 weeks of maintenance (UPA 15 mg [UPA15] once daily [QD] or UPA 30 mg [UPA30] QD) and entered the LTE, were analyzed. CS-free CR (Adapted Mayo score ≤ 2, with stool frequency subscore ≤ 1 and not greater than baseline, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1) was evaluated at LTE week 96 in clinical responders (per Adapted Mayo score) at maintenance week 0, without CS use at LTE week 96, that stayed on the same dose in the LTE (UPA15/15 and UPA30/30 groups). Data are presented as observed.
Results: Among patients in the overall population who entered the LTE, 78.5% (n=51/65) and 73.4% (80/109) in the UPA15/15 and UPA30/30 groups, respectively, achieved CS-free CR at LTE week 96 (Figure 1A). Additionally, among patients with CS use at induction baseline who entered the LTE, 75.0% (15/20) and 68.4% (26/38) in the UPA15/15 and UPA30/30 groups, respectively, achieved CS-free CR at LTE week 96. Maintenance of CS-free CR at LTE week 96 was achieved by 81.8% (45/55) and 83.3% (60/72) of patients in the UPA15/15 and UPA30/30 groups, respectively, in the overall population (Figure 1B). Additionally, maintenance of CS-free CR was achieved by 77.8% (14/18) and 80.0% (20/25) of patients in the UPA15/15 and UPA30/30 groups, respectively, with CS use at induction baseline.
Discussion: Although the number of patients analyzed was limited, most patients achieved and maintained CS-free CR through LTE week 96. These findings suggest the benefit of UPA as a long-term, CS-sparing treatment option for moderately to severely active UC.
References:
1. Danese S et al. Lancet 2022;399:2113–28
2. Raine T et al. J Crohn’s Colitis 2024;18(5):695–707
3. Colombel J-F et al. J Crohn's and Colitis. 2022;16(S1):i514
4. Vermeire S et al. Lancet Gastroenterol Hepatol 2023;8:976–89

Figure: Figure 1. CS-Free Clinical Remission per Adapted Mayo Score at LTE Week 96 of U-ACTIVATE by Baseline CS Use in Patients With Moderately to Severely Active UC.
AMS, Adapted Mayo score; BL, baseline; CI, confidence interval; CS, corticosteroid; LTE, long-term extension; QD, once daily; UC, ulcerative colitis; UPA, upadacitinib.
Includes UPA 45 mg QD 8-week induction responders who completed the 52-week maintenance.
Data are presented as observed.
Values above bars are percent of patients and values within the bars are n/n.
All data presented are from patients with clinical response per AMS at maintenance week 0.
AMS: the sum of the stool frequency subscore, rectal bleeding subscore, and endoscopic subscore.
Clinical remission by AMS: an AMS ≤ 2, with stool frequency subscore ≤ 1 and not greater than BL, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1.
BL CS use defined as patients with BL CS use entering the induction study (yes/no).
Maintenance of CS-free clinical remission per AMS at LTE week 96 based on patients who achieved CS-free clinical response at week 0 of maintenance.
AMS, Adapted Mayo score; BL, baseline; CI, confidence interval; CS, corticosteroid; LTE, long-term extension; QD, once daily; UC, ulcerative colitis; UPA, upadacitinib.
Includes UPA 45 mg QD 8-week induction responders who completed the 52-week maintenance.
Data are presented as observed.
Values above bars are percent of patients and values within the bars are n/n.
All data presented are from patients with clinical response per AMS at maintenance week 0.
AMS: the sum of the stool frequency subscore, rectal bleeding subscore, and endoscopic subscore.
Clinical remission by AMS: an AMS ≤ 2, with stool frequency subscore ≤ 1 and not greater than BL, rectal bleeding subscore = 0, and endoscopic subscore ≤ 1.
BL CS use defined as patients with BL CS use entering the induction study (yes/no).
Maintenance of CS-free clinical remission per AMS at LTE week 96 based on patients who achieved CS-free clinical response at week 0 of maintenance.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. BMS – Consultant. Boehringer Ingelheim – Consultant. Celgene Corporation – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Lilly – Consultant. Pfizer – Consultant. Prometheus Labs – Consultant. Sanofi – Consultant. Shire – Consultant. Takeda – Consultant.
Raja Atreya: AbbVie – Consultant, Honoraria. Abivax – Consultant, Speakers fees. Amgen – Consultant, Honoraria. Arena Pharmaceuticals – Consultant, Honoraria. AstraZeneca – Consultant, Speakers fees. Biogen – Consultant, Honoraria. Boehringer Ingelheim – Consultant, Honoraria. Bristol Myers Squibb – Consultant, Honoraria. Celgene – Consultant, Honoraria. Celltrion Healthcare – Consultant, Honoraria. Dr. Falk Pharma – Consultant, Honoraria. Ferring – Consultant, Honoraria. Fresenius Kabi – Consultant, Honoraria. Galapagos – Consultant, Honoraria. Gilead – Consultant, Honoraria. GSK – Consultant, Honoraria. InDex Pharmaceuticals – Consultant, Honoraria. Janssen – Consultant, Honoraria. Kiniksa Pharmaceuticals – Consultant, Honoraria. Merk Sharp & Dohme – Consultant, Honoraria. Novartis – Consultant, Honoraria. Pfizer – Consultant, Honoraria. Roche – Consultant, Honoraria. Samsung Bioepis – Consultant, Honoraria. Stelic – Consultant, Honoraria. Sterna Biologicals – Consultant, Honoraria. Takeda – Consultant, Honoraria. Tillotts – Consultant, Honoraria.
Hiroshi Nakase: AbbVie – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Celgene – Grant/Research Support. Daiichi Sankyo Company – Grant/Research Support. EA Pharma – Grant/Research Support. Hoya Group Pentax Medical – Grant/Research Support. Janssen – Grant/Research Support. JIMRO – Grant/Research Support. Kissei Pharmaceutical – Grant/Research Support. Kyorin Pharmaceutical – Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support. Mochida Pharmaceutical – Grant/Research Support. Nippon Kayaku – Grant/Research Support. Pfizer – Grant/Research Support. Takeda – Grant/Research Support. Zeria – Grant/Research Support.
Michelle Kujawski: AbbVie – Employee, Stock Options.
James Crooks: AbbVie – Employee, Stock Options.
Erica Cheng: AbbVie – Employee, Stock Options.
Justin Klaff: AbbVie – Employee, Stock Options.
Remo Panaccione: Élan – Consultant. Abbivax – Consultant. Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaking Fees. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker Fees. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker Fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker Fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker Fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Ferring – Advisory Committee/Board Member, Consultant, Speaker Fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker Fees. Galapagos – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Speaker Fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Bio – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speaker Fees. Lilly – Advisory Committee/Board Member, Consultant, Speaker Fees. Merck – Advisory Committee/Board Member, Consultant, Speaker Fees. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker Fees. Pandion Pharma – Advisory Committee/Board Member, Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speaker Fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker Fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker Fees. Satisfai Health – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speaker Fees. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speaker Fees. Theravance – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Marla Dubinsky, MD1, Raja Atreya, MD2, Hiroshi Nakase, MD3, Michelle Kujawski, PhD4, James Crooks, PhD5, Erica Cheng, PhD4, Justin Klaff, MD4, Remo Panaccione, MD6. P0849 - Corticosteroid-Free Remission Through 3 Years of Upadacitinib Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Data From the Phase 3 Long-Term Extension Study U-ACTIVATE, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
