Sunday Poster Session
Category: IBD
P0852 - Etrasimod for Moderately to Severely Active Crohn’s Disease: Results From the Extension Period of a Phase 2 Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Marla Dubinsky, MD
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Geert R. D'Haens, MD, PhD1, Marla C. Dubinsky, MD2, Laurent Peyrin-Biroulet, MD, PhD3, Silvio Danese, MD, PhD4, Bruce E.. Sands, MD, FACG2, Andres J. Yarur, MD5, Michael V. Chiorean, MD6, Irene Modesto, MD7, Diogo Branquinho, MD, MSc7, Aoibhinn McDonnell, PhD8, Maria Kudela, PhD9, Leonel Villa-Caballero, MD10, Guibao Gu, MD11, Huaming Tan, 7, Chinyu Su, MD12, Stefan Schreiber, MD13, Brian G.. Feagan, MD14, Séverine Vermeire, MD, PhD15
1Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 4Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 5Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA; 6Inflammatory Bowel Disease Center, Swedish Medical Center, Issaquah, WA; 7Pfizer Inc., New York, NY; 8Pfizer Ltd., Sandwich, England, United Kingdom; 9Pfizer Inc., Cambridge, MA; 10Pfizer Inc., San Diego, CA; 11Pfizer Inc., La Jolla, CA; 12Pfizer Inc., Collegeville, PA; 13University Hospital, Kiel, Schleswig-Holstein, Germany; 14Western University, London, ON, Canada; 15University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator. Within the phase 2 / 3 CULTIVATE trial (NCT04173273), we report extension data on the efficacy, safety, and tolerability of etrasimod in patients with moderately to severely active Crohn’s disease (CD) from substudy A, a phase 2, randomized, double-blind study.
Methods: Adults (18–80 years) who completed the 14-week (W) induction phase (data reported previously1) were eligible for the 52W extension period. Patients receiving etrasimod 3 mg during induction continued this treatment within the extension period, regardless of response. Etrasimod 2 mg responders at W14 continued treatment during the extension period, while nonresponders were re-randomized (1:1) to etrasimod 2 or 3 mg (Figure). Two analyses were performed based on two approaches for patients entering extension: treat-through (TT; outcomes reported for all randomized patients regardless of W14 response) and responders only (RO; outcomes reported for W14 responders only). Both used non-responder imputation. The primary endpoint was the achievement of endoscopic response (defined by endoscopic remission or ≥ 50% decrease in SES-CD) at Week 52; other endpoints included the achievement of clinical remission based on CDAI (< 150) and based on PRO2 (< 8) at Week 52 (Table). Treatment-emergent adverse events (TEAEs) were reported for the safety set (Figure).
Results: Patient disposition and baseline characteristics are shown in the Table and Figure. For the TT analysis, 14.3% and 19.5% of patients receiving 2 mg or 3 mg etrasimod, respectively, achieved endoscopic response, 28.6% and 34.1% achieved CDAI remission, and 19.0% and 36.6% achieved PRO2 remission at W52 (Table). Among W14 responders (RO), 23.1% and 33.3% of patients receiving 2 mg or 3 mg etrasimod, respectively, achieved endoscopic response, 46.2% and 54.2% achieved CDAI remission, and 30.8% and 58.3% achieved PRO2 remission at W52 (Table). Most TEAEs were mild or moderate. Nine TEAEs led to discontinuation; one was deemed treatment-related (Table). No deaths or TEAEs of macular edema or malignancies were reported.
Discussion: Both etrasimod doses remained well tolerated with no new safety signals. This non-placebo-controlled study suggests that etrasimod may be effective in moderately to severely active CD, with higher efficacy at the 3 mg dose. Placebo-controlled studies are ongoing.
Reference:
1. D’Haens G et al. Journal Crohns Colitis 2023; 17: i764–i765.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Geert R. D'Haens, MD, PhD1, Marla C. Dubinsky, MD2, Laurent Peyrin-Biroulet, MD, PhD3, Silvio Danese, MD, PhD4, Bruce E.. Sands, MD, FACG2, Andres J. Yarur, MD5, Michael V. Chiorean, MD6, Irene Modesto, MD7, Diogo Branquinho, MD, MSc7, Aoibhinn McDonnell, PhD8, Maria Kudela, PhD9, Leonel Villa-Caballero, MD10, Guibao Gu, MD11, Huaming Tan, 7, Chinyu Su, MD12, Stefan Schreiber, MD13, Brian G.. Feagan, MD14, Séverine Vermeire, MD, PhD15. P0852 - Etrasimod for Moderately to Severely Active Crohn’s Disease: Results From the Extension Period of a Phase 2 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 4Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 5Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center, Los Angeles, CA; 6Inflammatory Bowel Disease Center, Swedish Medical Center, Issaquah, WA; 7Pfizer Inc., New York, NY; 8Pfizer Ltd., Sandwich, England, United Kingdom; 9Pfizer Inc., Cambridge, MA; 10Pfizer Inc., San Diego, CA; 11Pfizer Inc., La Jolla, CA; 12Pfizer Inc., Collegeville, PA; 13University Hospital, Kiel, Schleswig-Holstein, Germany; 14Western University, London, ON, Canada; 15University Hospitals Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator. Within the phase 2 / 3 CULTIVATE trial (NCT04173273), we report extension data on the efficacy, safety, and tolerability of etrasimod in patients with moderately to severely active Crohn’s disease (CD) from substudy A, a phase 2, randomized, double-blind study.
Methods: Adults (18–80 years) who completed the 14-week (W) induction phase (data reported previously1) were eligible for the 52W extension period. Patients receiving etrasimod 3 mg during induction continued this treatment within the extension period, regardless of response. Etrasimod 2 mg responders at W14 continued treatment during the extension period, while nonresponders were re-randomized (1:1) to etrasimod 2 or 3 mg (Figure). Two analyses were performed based on two approaches for patients entering extension: treat-through (TT; outcomes reported for all randomized patients regardless of W14 response) and responders only (RO; outcomes reported for W14 responders only). Both used non-responder imputation. The primary endpoint was the achievement of endoscopic response (defined by endoscopic remission or ≥ 50% decrease in SES-CD) at Week 52; other endpoints included the achievement of clinical remission based on CDAI (< 150) and based on PRO2 (< 8) at Week 52 (Table). Treatment-emergent adverse events (TEAEs) were reported for the safety set (Figure).
Results: Patient disposition and baseline characteristics are shown in the Table and Figure. For the TT analysis, 14.3% and 19.5% of patients receiving 2 mg or 3 mg etrasimod, respectively, achieved endoscopic response, 28.6% and 34.1% achieved CDAI remission, and 19.0% and 36.6% achieved PRO2 remission at W52 (Table). Among W14 responders (RO), 23.1% and 33.3% of patients receiving 2 mg or 3 mg etrasimod, respectively, achieved endoscopic response, 46.2% and 54.2% achieved CDAI remission, and 30.8% and 58.3% achieved PRO2 remission at W52 (Table). Most TEAEs were mild or moderate. Nine TEAEs led to discontinuation; one was deemed treatment-related (Table). No deaths or TEAEs of macular edema or malignancies were reported.
Discussion: Both etrasimod doses remained well tolerated with no new safety signals. This non-placebo-controlled study suggests that etrasimod may be effective in moderately to severely active CD, with higher efficacy at the 3 mg dose. Placebo-controlled studies are ongoing.
Reference:
1. D’Haens G et al. Journal Crohns Colitis 2023; 17: i764–i765.

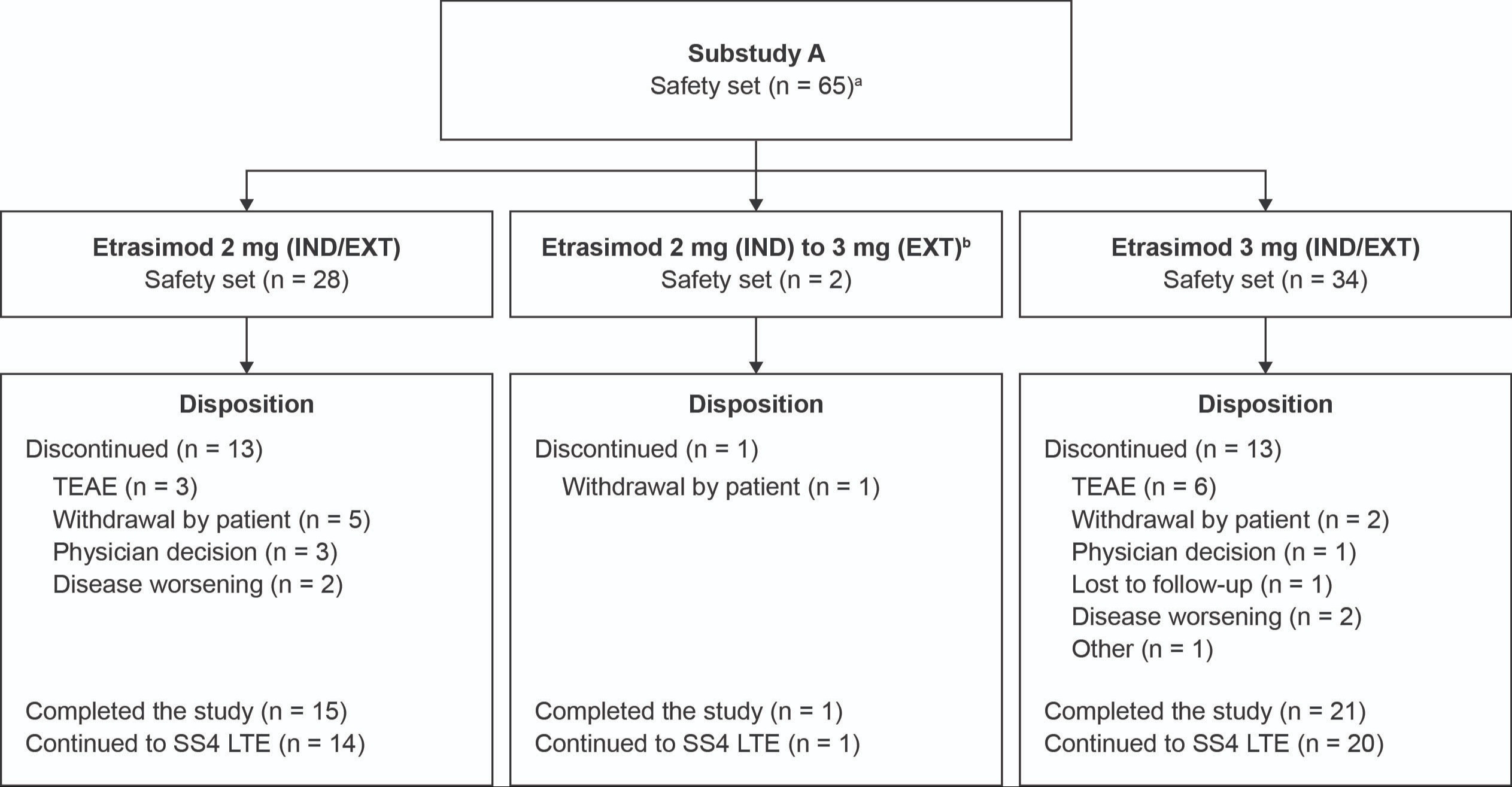
Figure: Figure. Patient disposition in the extension period.
The safety set (all randomized patients who received ≥ 1 dose) for the 3 mg group in the extension period included 37 patients (34 patients treated with 3 mg during IND and EXT, two patients treated with etrasimod 2 mg at IND who were re-randomized to 3 mg during EXT, and one patient that received placebo [randomized to placebo per a previous amendment of the study protocol, the patient was excluded in summary analyses of efficacy and safety]). The safety set for the 2 mg group includes 28 patients. Patients who completed 66 weeks of treatment in Substudy A were eligible to enroll in long-term extension (SS4 LTE).
[a]One patient received placebo during the IND period and etrasimod 3 mg QD during the EXT period.
[b]Two patients treated with etrasimod 2 mg QD during the IND period were nonresponders and were re-randomized to the 3 mg QD treatment group. These patients were counted as nonresponders in the 2 mg group in the TT design.
EXT, extension; IND, induction; N, number of patients in group; n, number of patients; QD, once daily; SS4 LTE, substudy 4 long-term extension; TEAE, treatment-emergent adverse event; TT, treat-through.
The safety set (all randomized patients who received ≥ 1 dose) for the 3 mg group in the extension period included 37 patients (34 patients treated with 3 mg during IND and EXT, two patients treated with etrasimod 2 mg at IND who were re-randomized to 3 mg during EXT, and one patient that received placebo [randomized to placebo per a previous amendment of the study protocol, the patient was excluded in summary analyses of efficacy and safety]). The safety set for the 2 mg group includes 28 patients. Patients who completed 66 weeks of treatment in Substudy A were eligible to enroll in long-term extension (SS4 LTE).
[a]One patient received placebo during the IND period and etrasimod 3 mg QD during the EXT period.
[b]Two patients treated with etrasimod 2 mg QD during the IND period were nonresponders and were re-randomized to the 3 mg QD treatment group. These patients were counted as nonresponders in the 2 mg group in the TT design.
EXT, extension; IND, induction; N, number of patients in group; n, number of patients; QD, once daily; SS4 LTE, substudy 4 long-term extension; TEAE, treatment-emergent adverse event; TT, treat-through.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Marla Dubinsky: AbbVie – Consultant. Abivax – Consultant. Arena – Consultant. AstraZeneca – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant. Pfizer Inc – Consultant. Prometheus Labs – Consultant. Takeda – Consultant.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Applied Molecular Transport – Consultant. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion Healthcare – Consultant. Dr Falk Pharma – Consultant. Eli Lilly and Company – Consultant. Enthera – Consultant. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant. Inotrem – Consultant. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant. Morphic – Consultant. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Speakers Bureau. Teladoc Health – Consultant. TiGenix – Consultant. UCB Inc. – Consultant. Vial – Consultant. Vifor – Consultant.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Andres Yarur: AbbVie – Consultant, Lecture Fees. Arena – Consultant. Bristol Myers Squibb – Consultant, Lecture Fees. Pfizer Inc – Consultant. Takeda – Consultant.
Michael Chiorean: AbbVie – Consultant, Speakers Bureau. Arena – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Medtronic – Consultant, Speakers Bureau. Novartis – Grant/Research Support. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Biosciences – Consultant. Takeda – Consultant.
Irene Modesto: Pfizer Inc – Employee, Stock Options.
Diogo Branquinho: Pfizer Inc – Employee, Stock Options.
Aoibhinn McDonnell: Pfizer Inc – Stock Options. Pfizer Ltd – Employee.
Maria Kudela: Pfizer Inc – Employee, Stock Options.
Leonel Villa-Caballero: Arena – Stock Options. Bristol Myers Squibb – Employee, Stock Options. Pfizer Inc – Was an employee of Pfizer Inc at the time of analysis.
Guibao Gu: Pfizer Inc – Employee, Stock Options.
Huaming Tan: Pfizer Inc – Employee, Stock Options.
Chinyu Su: Pfizer Inc – Employee, Stock Options.
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Advisory Committee/Board Member, Consultant. Applied Molecular Transport Inc – Advisory Committee/Board Member, Consultant. Arena Pharma – Consultant. Atomwise – Consultant. Avoro Capital Advisors – Consultant. Axio Research – Advisory Committee/Board Member. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boxer – Consultant. Celgene/Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celsius Therapeutics – Consultant. Connect BioPharma – Consultant, stock or other ownership interest. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 Capital – Advisory Committee/Board Member, Consultant. Equillium – Consultant. Ermium – Consultant. First Wave – Consultant. First Word Group – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Gossamer Pharma – Consultant, Stock Options. Hinge Bio – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. Immunic Therapeutics – Consultant. InDex Pharmaceuticals – Advisory Committee/Board Member, Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. Lenczner Slaght – Consultant, payment for expert testimony. LifeSci Capital – Consultant. Lilly – Advisory Committee/Board Member, Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Advisory Committee/Board Member, Consultant. Morgan Lewis – Consultant, payment for expert testimony. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Pandion Therapeutics – Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. REDX – Advisory Committee/Board Member, Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. Ventyx Biosciences – Consultant. VHSquared Ltd – Consultant. Viatris – Consultant. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. Abivax – Consultant. AbolerlsPharma – Consultant. AgomAb – Consultant. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. BioraTherapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Cytoki Pharma – Consultant. Dr Falk Pharma – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Galapagos – Consultant, Grant/Research Support. Genentech Roche – Consultant. Gilead – Consultant. GSK – Consultant. Hospira – Consultant. J&J – Consultant, Grant/Research Support. Janssen – Consultant. lmidomics – Consultant. Materia Prima – Consultant. Mestag Therapeutics – Consultant. Microbiotica – Consultant. MiroBio – Consultant. Morphic – Consultant. MrMHealth – Consultant. MSD – Consultant. Mundipharma – Consultant. Pfizer Inc – Consultant, Grant/Research Support. Prodigest – Consultant. Progenity – Consultant. Prometheus – Consultant. Robarts Clinical Trials – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Consultant. Tillots Pharma AG – Consultant. VectivBio – Consultant. Ventyx – Consultant. Zealand Pharma – Consultant.
Geert R. D'Haens, MD, PhD1, Marla C. Dubinsky, MD2, Laurent Peyrin-Biroulet, MD, PhD3, Silvio Danese, MD, PhD4, Bruce E.. Sands, MD, FACG2, Andres J. Yarur, MD5, Michael V. Chiorean, MD6, Irene Modesto, MD7, Diogo Branquinho, MD, MSc7, Aoibhinn McDonnell, PhD8, Maria Kudela, PhD9, Leonel Villa-Caballero, MD10, Guibao Gu, MD11, Huaming Tan, 7, Chinyu Su, MD12, Stefan Schreiber, MD13, Brian G.. Feagan, MD14, Séverine Vermeire, MD, PhD15. P0852 - Etrasimod for Moderately to Severely Active Crohn’s Disease: Results From the Extension Period of a Phase 2 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
