Sunday Poster Session
Category: IBD
P0858 - Comparing Ustekinumab and Vedolizumab in Inflammatory Bowel Disease Patients Exposed to Anti-TNF Agents: A Propensity-Matched, Retrospective Cohort Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- FA
Feyzullah Aksan, MD
Renaissance School of Medicine, Stony Brook University
Port Jefferson Station, NY
Presenting Author(s)
Feyzullah Aksan, MD1, Layla Barrera, DO2, Thomas Ullman, MD3
1Renaissance School of Medicine, Stony Brook University, Port Jefferson Station, NY; 2Stony Brook University Hospital, Port Jefferson Station, NY; 3Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
Introduction: Anti-TNF agents are commonly used to treat Inflammatory Bowel Disease (IBD), but loss of response due to immunogenicity, intolerance, and other reasons can prompt a switch to alternative therapies like ustekinumab (UST), IL12/IL23 antagonist, or vedolizumab (VDZ) ,α4β7 integrin antagonist . While some post hoc-reports compare UST and VDZ for achieving remission in IBD patients, there needs to be more evaluation regarding critical outcomes such as mortality, disease-related complications, and surgeries. Our retrospective study aims to bridge this gap
Methods: We used TriNetX, a multi-institutional database, for a retrospective cohort study comparing UST and VDZ in patients with Crohn’s disease (CD) or ulcerative colitis (UC) previously exposed to anti-TNF agents. Using ICD-10 codes, patients aged ≥18 years with CD or UC and prior anti-TNF exposure were categorized into two cohorts: VDZ- or UST-recivied post index event. The index event was the CD or UC diagnosis between January 1, 2014, and May 1, 2024. We excluded patients with prior colectomy or ileal resection. We employed 1:1 propensity score matching (PSM) based on demographics, age, gender, comorbidities, IBD subtypes, and concurrent medications. Clinical outcomes within two years post-index event, including mortality, colectomy, ileal resection, abscess, fistula, steroid use, and healthcare resource utilization (HRU; hospital/office visits), were evaluated. Cox regression estimated hazard ratios (HR) with 95% CI, using α < 0.05 for significance.
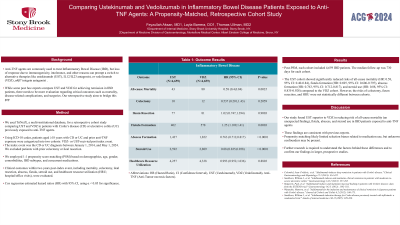
Results: Post-PSM, each cohort included 4,659 IBD patients. The median follow-up was 730 days for each cohort. The UST cohort showed significantly reduced risks of all-cause mortality (HR: 0.58, 95% CI: 0.40-0.84), fistula formation (HR: 0.695, 95% CI: 0.606-0.797), abscess formation (HR: 0.763, 95% CI: 0.712-0.817), and steroid use (HR: 0.88, 95% CI: 0.835-0.928) compared to the VDZ cohort. However, the risks of colectomy, ileum resection, and HRU were not statistically different between cohorts.
Discussion: Our study found UST superior to VDZ in reducing risk of all-cause mortality (an unexpected finding), fistula, abscess, and steroid use in IBD patients exposed to anti-TNF agents. These findings are consistent with previous reports. Propensity matching likely limited selection biases related to medication use, but unknown confounders may be present. Further research is required to understand the factors behind these differences and to confirm our findings in larger, prospective studies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Feyzullah Aksan, MD1, Layla Barrera, DO2, Thomas Ullman, MD3. P0858 - Comparing Ustekinumab and Vedolizumab in Inflammatory Bowel Disease Patients Exposed to Anti-TNF Agents: A Propensity-Matched, Retrospective Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Renaissance School of Medicine, Stony Brook University, Port Jefferson Station, NY; 2Stony Brook University Hospital, Port Jefferson Station, NY; 3Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
Introduction: Anti-TNF agents are commonly used to treat Inflammatory Bowel Disease (IBD), but loss of response due to immunogenicity, intolerance, and other reasons can prompt a switch to alternative therapies like ustekinumab (UST), IL12/IL23 antagonist, or vedolizumab (VDZ) ,α4β7 integrin antagonist . While some post hoc-reports compare UST and VDZ for achieving remission in IBD patients, there needs to be more evaluation regarding critical outcomes such as mortality, disease-related complications, and surgeries. Our retrospective study aims to bridge this gap
Methods: We used TriNetX, a multi-institutional database, for a retrospective cohort study comparing UST and VDZ in patients with Crohn’s disease (CD) or ulcerative colitis (UC) previously exposed to anti-TNF agents. Using ICD-10 codes, patients aged ≥18 years with CD or UC and prior anti-TNF exposure were categorized into two cohorts: VDZ- or UST-recivied post index event. The index event was the CD or UC diagnosis between January 1, 2014, and May 1, 2024. We excluded patients with prior colectomy or ileal resection. We employed 1:1 propensity score matching (PSM) based on demographics, age, gender, comorbidities, IBD subtypes, and concurrent medications. Clinical outcomes within two years post-index event, including mortality, colectomy, ileal resection, abscess, fistula, steroid use, and healthcare resource utilization (HRU; hospital/office visits), were evaluated. Cox regression estimated hazard ratios (HR) with 95% CI, using α < 0.05 for significance.
Results: Post-PSM, each cohort included 4,659 IBD patients. The median follow-up was 730 days for each cohort. The UST cohort showed significantly reduced risks of all-cause mortality (HR: 0.58, 95% CI: 0.40-0.84), fistula formation (HR: 0.695, 95% CI: 0.606-0.797), abscess formation (HR: 0.763, 95% CI: 0.712-0.817), and steroid use (HR: 0.88, 95% CI: 0.835-0.928) compared to the VDZ cohort. However, the risks of colectomy, ileum resection, and HRU were not statistically different between cohorts.
Discussion: Our study found UST superior to VDZ in reducing risk of all-cause mortality (an unexpected finding), fistula, abscess, and steroid use in IBD patients exposed to anti-TNF agents. These findings are consistent with previous reports. Propensity matching likely limited selection biases related to medication use, but unknown confounders may be present. Further research is required to understand the factors behind these differences and to confirm our findings in larger, prospective studies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Feyzullah Aksan indicated no relevant financial relationships.
Layla Barrera indicated no relevant financial relationships.
Thomas Ullman: BMS – Consultant. Pfizer – Advisor or Review Panel Member, Grant/Research Support.
Feyzullah Aksan, MD1, Layla Barrera, DO2, Thomas Ullman, MD3. P0858 - Comparing Ustekinumab and Vedolizumab in Inflammatory Bowel Disease Patients Exposed to Anti-TNF Agents: A Propensity-Matched, Retrospective Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
