Sunday Poster Session
Category: IBD
P0859 - Impact of Body Mass Index on the Effectiveness of Biologic Therapies in Inflammatory Bowel Disease: A Retrospective, Propensity Score-Matched Cohort Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- FA
Feyzullah Aksan, MD
Renaissance School of Medicine, Stony Brook University
Port Jefferson Station, NY
Presenting Author(s)
Feyzullah Aksan, MD1, Farah Monzur, MD, FACG2
1Renaissance School of Medicine, Stony Brook University, Port Jefferson Station, NY; 2Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Introduction: Biologics have transformed inflammatory bowel disease (IBD) management, but the responses can be influenced by multiple factors. High body mass index (BMI) is identified as a risk factor for increased drug clearance. While small cohort studies have evaluated the impact of BMI on infliximab in IBD patients, there are no studies assessing the impact of BMI on other anti-TNFs and biologics in IBD patients. We conducted a retrospective study to address this gap.
Methods: We conducted this retrospective cohort study using TriNetX, a multi-institutional database, to assess the impact of BMI on the effectiveness of biologics in IBD. Patients, ≥18 years old, with Crohn’s Disease (CD) or Ulcerative Colitis (UC) before May 1, 2022, were divided into three groups based on biologic use: anti-TNFs, anti-integrin (VDZ), and interleukin 12/23 inhibitors (UST). Patients with colectomy or ileal resections were excluded. For each group, four cohorts were created based on BMI: Normal Weight (NW, BMI 18.5-24.9), Underweight (UW, BMI < 18.5), Overweight (OW, BMI 25-29.9), and Obese (OB, BMI >30). Patients with UW, OW, and OB were compared to NW within each biologic class. 1:1 propensity score matching (PSM) based on demographics, age, gender, comorbidities, IBD subtypes, and concurrent IBD medications was employed. Clinical outcomes within 2 years, including colectomy, ileum resection, abscess, fistula, and steroid use, were evaluated. Hazard Ratio (HR) and 95% CI for risk assessment, with α < 0.05 for significance.
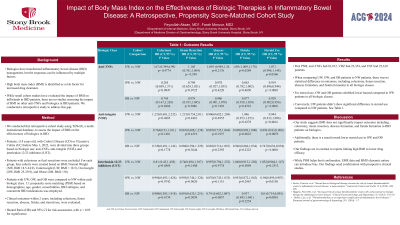
Results: Post PSM, anti-TNFs had 83,012, VDZ had 22,354, and UST had 22,610 patients. When comparing UW, OW, and OB patients to NW patients, there was no statistical difference in outcomes, including colectomy, ileum resection, abscess formation, and fistula formation in all biologic classes. For steroid use, OW and OB patients exhibited lower hazard compared to NW patients in all biologic classes. Conversely, UW patients didn’t show significant difference in steroid use compared to NW patients. See Table 1.
Discussion: Our study suggests BMI does not significantly impact outcomes including, colectomy, ileum resection, abscess formation, and fistula formation in IBD patients on biologics. Additionally, there is a trend toward lower steroid use in OW and OB patients. Our findings are in contrast to reports linking high BMI to lower drug efficacy. While PSM helps limit confounders, EMR data and BMI's dynamic nature can introduce bias. Our findings need confirmation with prospective clinical studies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Feyzullah Aksan, MD1, Farah Monzur, MD, FACG2. P0859 - Impact of Body Mass Index on the Effectiveness of Biologic Therapies in Inflammatory Bowel Disease: A Retrospective, Propensity Score-Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Renaissance School of Medicine, Stony Brook University, Port Jefferson Station, NY; 2Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Introduction: Biologics have transformed inflammatory bowel disease (IBD) management, but the responses can be influenced by multiple factors. High body mass index (BMI) is identified as a risk factor for increased drug clearance. While small cohort studies have evaluated the impact of BMI on infliximab in IBD patients, there are no studies assessing the impact of BMI on other anti-TNFs and biologics in IBD patients. We conducted a retrospective study to address this gap.
Methods: We conducted this retrospective cohort study using TriNetX, a multi-institutional database, to assess the impact of BMI on the effectiveness of biologics in IBD. Patients, ≥18 years old, with Crohn’s Disease (CD) or Ulcerative Colitis (UC) before May 1, 2022, were divided into three groups based on biologic use: anti-TNFs, anti-integrin (VDZ), and interleukin 12/23 inhibitors (UST). Patients with colectomy or ileal resections were excluded. For each group, four cohorts were created based on BMI: Normal Weight (NW, BMI 18.5-24.9), Underweight (UW, BMI < 18.5), Overweight (OW, BMI 25-29.9), and Obese (OB, BMI >30). Patients with UW, OW, and OB were compared to NW within each biologic class. 1:1 propensity score matching (PSM) based on demographics, age, gender, comorbidities, IBD subtypes, and concurrent IBD medications was employed. Clinical outcomes within 2 years, including colectomy, ileum resection, abscess, fistula, and steroid use, were evaluated. Hazard Ratio (HR) and 95% CI for risk assessment, with α < 0.05 for significance.
Results: Post PSM, anti-TNFs had 83,012, VDZ had 22,354, and UST had 22,610 patients. When comparing UW, OW, and OB patients to NW patients, there was no statistical difference in outcomes, including colectomy, ileum resection, abscess formation, and fistula formation in all biologic classes. For steroid use, OW and OB patients exhibited lower hazard compared to NW patients in all biologic classes. Conversely, UW patients didn’t show significant difference in steroid use compared to NW patients. See Table 1.
Discussion: Our study suggests BMI does not significantly impact outcomes including, colectomy, ileum resection, abscess formation, and fistula formation in IBD patients on biologics. Additionally, there is a trend toward lower steroid use in OW and OB patients. Our findings are in contrast to reports linking high BMI to lower drug efficacy. While PSM helps limit confounders, EMR data and BMI's dynamic nature can introduce bias. Our findings need confirmation with prospective clinical studies.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Feyzullah Aksan indicated no relevant financial relationships.
Farah Monzur: Gather-Ed – Consultant, Speakers Bureau. Medtronic (Covidien) – Consultant. Prometheus – Consultant.
Feyzullah Aksan, MD1, Farah Monzur, MD, FACG2. P0859 - Impact of Body Mass Index on the Effectiveness of Biologic Therapies in Inflammatory Bowel Disease: A Retrospective, Propensity Score-Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
