Sunday Poster Session
Category: IBD
P0862 - Vedolizumab Monotherapy Vs Vedolizumab Combined with Immunomodulators in Inflammatory Bowel Disease: A Propensity-Matched, Retrospective Cohort Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- FA
Feyzullah Aksan, MD
Renaissance School of Medicine, Stony Brook University
Port Jefferson Station, NY
Presenting Author(s)
Feyzullah Aksan, MD1, Layla Barrera, DO2, Thomas Ullman, MD3
1Renaissance School of Medicine, Stony Brook University, Port Jefferson Station, NY; 2Stony Brook University Hospital, Port Jefferson Station, NY; 3Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
Introduction: Patients with inflammatory bowel disease (IBD) often receive treatment with anti-TNF, sometimes in combination with immunomodulators (IMMs) methotrexate or thiopurines. When patients develop loss of response or intolerance to anti-TNF therapy, they often switch to different therapeutic classes while continuing IMMs if tolerated. Although there are established benefits of combining anti-TNF and IMMs, it remains uncertain whether similar benefits exist with concomitant use of vedolizumab (VDZ) and IMMs. This study aims to provide clarity on the effectiveness of combining VDZ with IMMs in IBD treatment.
Methods: We conducted a retrospective cohort study using TriNetX, a multi-institutional database, to compare effectiveness of VDZ alone to VDZ with IMM use. Using ICD-10 codes, we identified patients, ≥18 years old, with Crohn’s disease (CD) or ulcerative colitis (UC) between January 2014 and January 2022.We excluded patients with colectomy or ileal resection. Two cohorts are created; VDZ+ IMM or VDZ alone after CD or UC diagnosis. We employed 1:1 propensity score match (PSM) based on demographics, age, gender, comorbidities, IBD subtypes, and other IBD medications. We compared the 3-year risks of all-cause mortality, colectomy, ileal resection, abscess formation, fistula formation, steroid us, diffuse Large B-Cell lymphoma (DLBCL), opportunistic infections. We calculated hazard ratios (HR) and 95% Confidence Intervals (CI) to determine the effect of IMM use on outcomes with a significance level of α < 0.05.
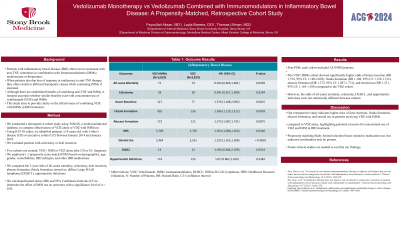
Results: Post-PSM, each cohort included 3,819 IBD patients. The VDZ+IMMs cohort showed significantly higher odds of ileum resection (HR 1.576, 95% CI: 1.188-2.092), fistula formation (HR 1.306, 95% CI: 1.129-1.512), abscess formation (HR 1.372, 95% CI: 1.087-1.731), and steroid use (HR 1.231, 95% CI: 1.165-1.308) compared to the VDZ cohort. However, the odds of all-cause mortality, colectomy, DLBCL, and opportunistic infections were not statistically different between cohorts.
Discussion: Our retrospective study indicates higher risks of ileal resection, fistula formation, abscess formation, and steroid use in patients receiving VDZ with IMMs. compared to VDZ alone, highlighting potential concerns for concomitant use of VDZ and IMM in IBD treatment.Propensity matching likely limited selection biases related to medication use, but unknown confounders may be present. Future clinical studies are needed to confirm our findings.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Feyzullah Aksan, MD1, Layla Barrera, DO2, Thomas Ullman, MD3. P0862 - Vedolizumab Monotherapy Vs Vedolizumab Combined with Immunomodulators in Inflammatory Bowel Disease: A Propensity-Matched, Retrospective Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Renaissance School of Medicine, Stony Brook University, Port Jefferson Station, NY; 2Stony Brook University Hospital, Port Jefferson Station, NY; 3Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
Introduction: Patients with inflammatory bowel disease (IBD) often receive treatment with anti-TNF, sometimes in combination with immunomodulators (IMMs) methotrexate or thiopurines. When patients develop loss of response or intolerance to anti-TNF therapy, they often switch to different therapeutic classes while continuing IMMs if tolerated. Although there are established benefits of combining anti-TNF and IMMs, it remains uncertain whether similar benefits exist with concomitant use of vedolizumab (VDZ) and IMMs. This study aims to provide clarity on the effectiveness of combining VDZ with IMMs in IBD treatment.
Methods: We conducted a retrospective cohort study using TriNetX, a multi-institutional database, to compare effectiveness of VDZ alone to VDZ with IMM use. Using ICD-10 codes, we identified patients, ≥18 years old, with Crohn’s disease (CD) or ulcerative colitis (UC) between January 2014 and January 2022.We excluded patients with colectomy or ileal resection. Two cohorts are created; VDZ+ IMM or VDZ alone after CD or UC diagnosis. We employed 1:1 propensity score match (PSM) based on demographics, age, gender, comorbidities, IBD subtypes, and other IBD medications. We compared the 3-year risks of all-cause mortality, colectomy, ileal resection, abscess formation, fistula formation, steroid us, diffuse Large B-Cell lymphoma (DLBCL), opportunistic infections. We calculated hazard ratios (HR) and 95% Confidence Intervals (CI) to determine the effect of IMM use on outcomes with a significance level of α < 0.05.
Results: Post-PSM, each cohort included 3,819 IBD patients. The VDZ+IMMs cohort showed significantly higher odds of ileum resection (HR 1.576, 95% CI: 1.188-2.092), fistula formation (HR 1.306, 95% CI: 1.129-1.512), abscess formation (HR 1.372, 95% CI: 1.087-1.731), and steroid use (HR 1.231, 95% CI: 1.165-1.308) compared to the VDZ cohort. However, the odds of all-cause mortality, colectomy, DLBCL, and opportunistic infections were not statistically different between cohorts.
Discussion: Our retrospective study indicates higher risks of ileal resection, fistula formation, abscess formation, and steroid use in patients receiving VDZ with IMMs. compared to VDZ alone, highlighting potential concerns for concomitant use of VDZ and IMM in IBD treatment.Propensity matching likely limited selection biases related to medication use, but unknown confounders may be present. Future clinical studies are needed to confirm our findings.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Feyzullah Aksan indicated no relevant financial relationships.
Layla Barrera indicated no relevant financial relationships.
Thomas Ullman: BMS – Consultant. Pfizer – Advisor or Review Panel Member, Grant/Research Support.
Feyzullah Aksan, MD1, Layla Barrera, DO2, Thomas Ullman, MD3. P0862 - Vedolizumab Monotherapy Vs Vedolizumab Combined with Immunomodulators in Inflammatory Bowel Disease: A Propensity-Matched, Retrospective Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
