Sunday Poster Session
Category: IBD
P0942 - Efficacy and Safety of Interleukin-12, 23 Inhibitors vs Interleukin-23 inhibitors for Moderate to Severe Ulcerative Colitis: A Systematic Review and Network Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Ravi Teja Pasam, MBBS, MPH
Wentworth-Douglass Hospital
Portsmouth, NH
Presenting Author(s)
Ravi Teja Pasam, MBBS, MPH1, Misha Gautam, MD2, Sruthi Venugopalan, MD3, Babu Mohan, MD4, Udaykumar Navaneethan, MD5
1Wentworth-Douglass Hospital, Portsmouth, NH; 2University of Missouri - Kansas City School of Medicine, Kansas City, MO; 3University of Nevada Reno School of Medicine, Reno, NV; 4Orlando Gastroenterology PA, Orlando, FL; 5Orlando Health, Digestive Health Institute, Orlando, FL
Introduction: Interleukin 23 (IL-23) inhibitors have been shown to have superior efficacy compared to interleukin - 12, 23 (IL-12,23) inhibitors in patients with psoriasis. It is unclear whether this translates to patients with moderate to severe ulcerative colitis (UC). A systematic review and network meta-analysis (NMA) of clinical drug trials was performed to answer this question.
Methods: Randomized controlled trials (RCTs) comparing IL-23 or IL-12/23 inhibitors to placebo or to each other in patients with moderate to severe UC were included. Primary outcomes were clinical response and clinical remission in the induction phase. Secondary outcomes included clinical response and clinical remission in the maintenance phase, endoscopic and safety outcomes in both induction and maintenance phases. A random-effects frequentist NMA was conducted. Subgroup analysis was performed in biologic-naive and biologic-experienced patients wherever possible.
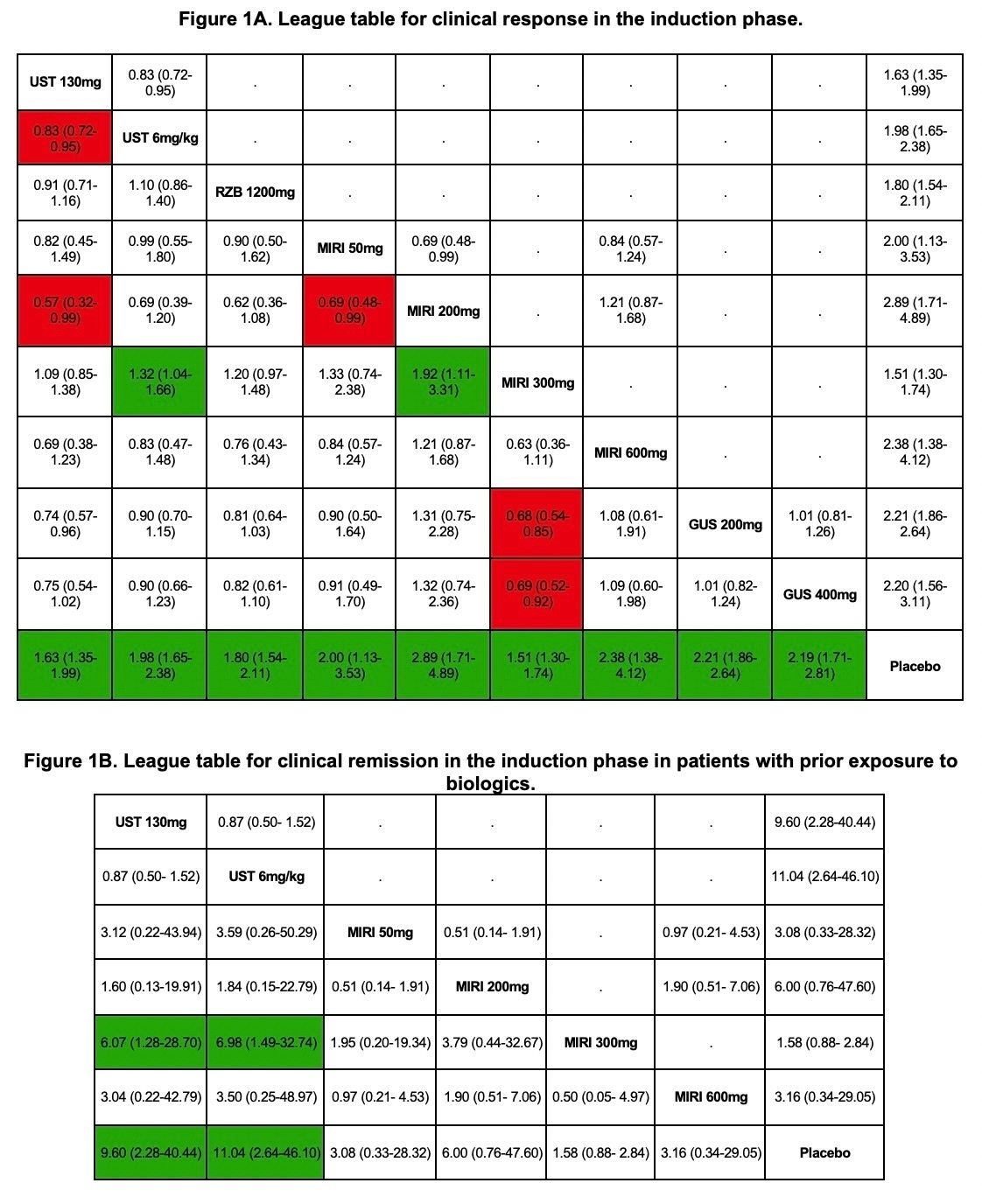
Results: Seven studies (4,361 patients) were analyzed. Mean age of the patients was 42.1 years [Standard deviation : 13.9] and 40% of them were women. Ustekinumab 6 mg/kg demonstrated superior efficacy compared to Mirikizumab 300 mg in terms of clinical response (Risk ratio [RR]: 1.32; 95% confidence intervals [95% CI]: 1.04, 1.66) (Figure 1A). Ustekinumab 6 mg/kg was associated with higher clinical remission rate compared to Mirikizumab 300 mg in the induction phase among patients with prior biologic exposure (RR: 6.98; 95% CI: 1.49, 32.74) (Figure 1B). There were no significant differences between Ustekinumab and IL-23 inhibitors for the secondary and safety outcomes.
Discussion: Ustekinumab should be considered over Mirikizumab for patients with moderate to severe UC, especially in patients with prior exposure to biologics. The safety profile of IL-23 inhibitors appears to be comparable to that of IL-12,23 inhibitors. Phase III trial data are required for IL-23 inhibitors other than Mirikizumab. Head-to-head RCTs are necessary to assess the relative efficacy of IL-12,23 vs IL-23 inhibitors.
Disclosures:
Ravi Teja Pasam, MBBS, MPH1, Misha Gautam, MD2, Sruthi Venugopalan, MD3, Babu Mohan, MD4, Udaykumar Navaneethan, MD5. P0942 - Efficacy and Safety of Interleukin-12, 23 Inhibitors vs Interleukin-23 inhibitors for Moderate to Severe Ulcerative Colitis: A Systematic Review and Network Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Wentworth-Douglass Hospital, Portsmouth, NH; 2University of Missouri - Kansas City School of Medicine, Kansas City, MO; 3University of Nevada Reno School of Medicine, Reno, NV; 4Orlando Gastroenterology PA, Orlando, FL; 5Orlando Health, Digestive Health Institute, Orlando, FL
Introduction: Interleukin 23 (IL-23) inhibitors have been shown to have superior efficacy compared to interleukin - 12, 23 (IL-12,23) inhibitors in patients with psoriasis. It is unclear whether this translates to patients with moderate to severe ulcerative colitis (UC). A systematic review and network meta-analysis (NMA) of clinical drug trials was performed to answer this question.
Methods: Randomized controlled trials (RCTs) comparing IL-23 or IL-12/23 inhibitors to placebo or to each other in patients with moderate to severe UC were included. Primary outcomes were clinical response and clinical remission in the induction phase. Secondary outcomes included clinical response and clinical remission in the maintenance phase, endoscopic and safety outcomes in both induction and maintenance phases. A random-effects frequentist NMA was conducted. Subgroup analysis was performed in biologic-naive and biologic-experienced patients wherever possible.
Results: Seven studies (4,361 patients) were analyzed. Mean age of the patients was 42.1 years [Standard deviation : 13.9] and 40% of them were women. Ustekinumab 6 mg/kg demonstrated superior efficacy compared to Mirikizumab 300 mg in terms of clinical response (Risk ratio [RR]: 1.32; 95% confidence intervals [95% CI]: 1.04, 1.66) (Figure 1A). Ustekinumab 6 mg/kg was associated with higher clinical remission rate compared to Mirikizumab 300 mg in the induction phase among patients with prior biologic exposure (RR: 6.98; 95% CI: 1.49, 32.74) (Figure 1B). There were no significant differences between Ustekinumab and IL-23 inhibitors for the secondary and safety outcomes.
Discussion: Ustekinumab should be considered over Mirikizumab for patients with moderate to severe UC, especially in patients with prior exposure to biologics. The safety profile of IL-23 inhibitors appears to be comparable to that of IL-12,23 inhibitors. Phase III trial data are required for IL-23 inhibitors other than Mirikizumab. Head-to-head RCTs are necessary to assess the relative efficacy of IL-12,23 vs IL-23 inhibitors.

Figure: League tables comparing the outcomes of Interleukin-12,23 inhibitors to Interleukin-23 inhibitors with risk ratios and 95% confidence intervals. Comparisons should be read from left (active agent) to right (control arm). Risk ratios above the diagonal represent direct pairwise meta-analysis. Risk ratios below the diagonal represent network meta-analysis. Risk ratios highlighted in green indicate favorable outcomes. Risk ratios highlighted in red indicate unfavorable outcome. UST - Ustekinumab; RZB - Risankizumab; MIRI - Mirikizumab; GUS - Guselkumab.
Disclosures:
Ravi Teja Pasam indicated no relevant financial relationships.
Misha Gautam indicated no relevant financial relationships.
Sruthi Venugopalan indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
Udaykumar Navaneethan: AbbVie – Consultant, Speakers Bureau. BMS – Speakers Bureau. Janssen – Consultant, Speakers Bureau. Lily – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Takeda – Speakers Bureau.
Ravi Teja Pasam, MBBS, MPH1, Misha Gautam, MD2, Sruthi Venugopalan, MD3, Babu Mohan, MD4, Udaykumar Navaneethan, MD5. P0942 - Efficacy and Safety of Interleukin-12, 23 Inhibitors vs Interleukin-23 inhibitors for Moderate to Severe Ulcerative Colitis: A Systematic Review and Network Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
