Sunday Poster Session
Category: IBD
P0965 - Efficacy and Safety of Vedolizumab in Chronic Pouchitis: A Systematic Review and Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Nihal Ijaz I. Khan, MD
AdventHealth Medical Group, AdventHealth
Orlando, FL
Presenting Author(s)
Nihal I. Khan, MD1, Nouman Shafique, MD1, Syed Hamaad Rahman, DO2, Aimen Farooq, MD3, Abu Hurairah, MD1
1AdventHealth Medical Group, AdventHealth, Orlando, FL; 2Methodist Dallas Medical Center, Irving, TX; 3AdventHealth, Orlando, FL
Introduction: Patients with proctocolectomy routinely undergo the formation of an ileal pouch-anal anastomosis (IPAA). Inflammation of the pouch is a common long-term complication of this procedure. Over the years, multiple therapies including antibiotics and biologics have been employed in treating chronic inflammation of the pouch. In this systematic review and meta-analysis, we aim to study the efficacy and safety of using vedolizumab to treat chronic pouchitis.
Methods: A systematic review of the literature from MEDLINE, EMBASE and Scopus was conducted from inception to June 2024, for studies reporting on the use of vedolizumab in the treatment of chronic pouchitis OR chronic inflammation of the pouch. Outcomes of interest were clinical and endoscopic response per the study definition, disease remission per the study definition, and adverse events at the initial follow-up period. Standard meta-analysis methods were followed using the random-effects model. Heterogeneity was assessed using the I2% statistics.
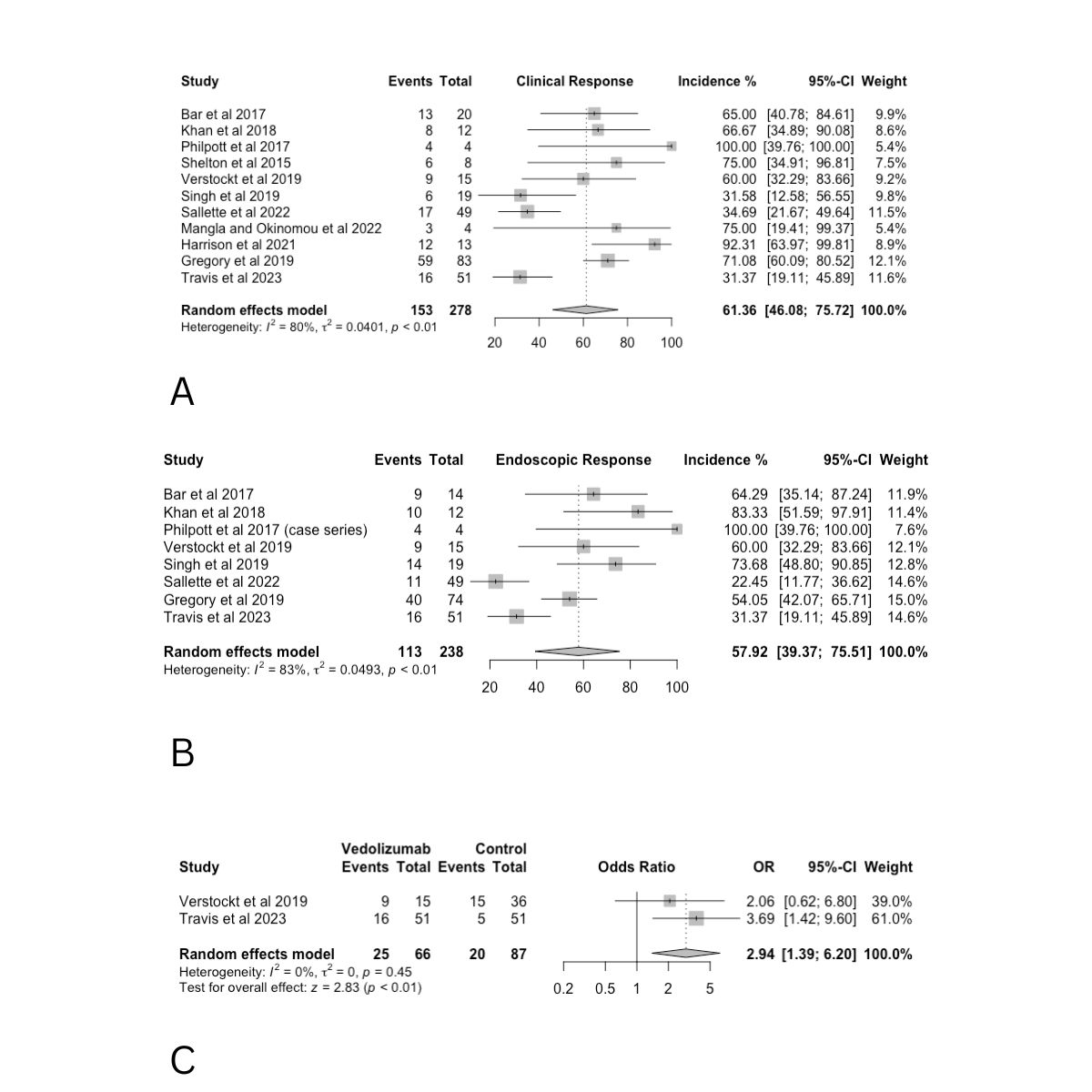
Results: 12 studies were identified; this included 1 multicenter randomized controlled trial, 7 retrospective studies including 4 multicenter and 3 single-center studies, 1 single-center prospective observational study, and 3 case series. The pooled rate of clinical response involving 11 studies and a total of 278 patients was 61.36% (95% CI 46.08-75.72, p< 0.01, I2:80%). The pooled rate of endoscopic response involving 8 studies and a total of 238 patients was 57.92% (95% CI 39.37-75.51, p< 0.01, I2:83%). We also compared disease remission between vedolizumab with either placebo or adalimumab plus infliximab among two studies and found the odd’s ratio was 2.94 (95% CI 1.39-6.20, p< 0.01, I2:0%).
The pooled rate of adverse events among 278 patients in 11 studies was 61.36% (95% CI 46.08-75.72, p< 0.01, I2:80%). Results are summarized in Figure 1.
Discussion: Our findings suggest that vedolizumab shows promise in treating chronic pouchitis, with clinical and endoscopic response rates of 61.36% and 57.92%, respectively. The comparison of disease remission rates for vedolizumab versus placebo or adalimumab plus infliximab suggests that vedolizumab may be superior to other biologic agents. The high adverse event rate of 61.36% highlights the necessity for careful monitoring. Overall, vedolizumab appears effective, but standardized research and further studies are essential to confirm its long-term efficacy and safety, enabling better management of chronic pouchitis.
Disclosures:
Nihal I. Khan, MD1, Nouman Shafique, MD1, Syed Hamaad Rahman, DO2, Aimen Farooq, MD3, Abu Hurairah, MD1. P0965 - Efficacy and Safety of Vedolizumab in Chronic Pouchitis: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1AdventHealth Medical Group, AdventHealth, Orlando, FL; 2Methodist Dallas Medical Center, Irving, TX; 3AdventHealth, Orlando, FL
Introduction: Patients with proctocolectomy routinely undergo the formation of an ileal pouch-anal anastomosis (IPAA). Inflammation of the pouch is a common long-term complication of this procedure. Over the years, multiple therapies including antibiotics and biologics have been employed in treating chronic inflammation of the pouch. In this systematic review and meta-analysis, we aim to study the efficacy and safety of using vedolizumab to treat chronic pouchitis.
Methods: A systematic review of the literature from MEDLINE, EMBASE and Scopus was conducted from inception to June 2024, for studies reporting on the use of vedolizumab in the treatment of chronic pouchitis OR chronic inflammation of the pouch. Outcomes of interest were clinical and endoscopic response per the study definition, disease remission per the study definition, and adverse events at the initial follow-up period. Standard meta-analysis methods were followed using the random-effects model. Heterogeneity was assessed using the I2% statistics.
Results: 12 studies were identified; this included 1 multicenter randomized controlled trial, 7 retrospective studies including 4 multicenter and 3 single-center studies, 1 single-center prospective observational study, and 3 case series. The pooled rate of clinical response involving 11 studies and a total of 278 patients was 61.36% (95% CI 46.08-75.72, p< 0.01, I2:80%). The pooled rate of endoscopic response involving 8 studies and a total of 238 patients was 57.92% (95% CI 39.37-75.51, p< 0.01, I2:83%). We also compared disease remission between vedolizumab with either placebo or adalimumab plus infliximab among two studies and found the odd’s ratio was 2.94 (95% CI 1.39-6.20, p< 0.01, I2:0%).
The pooled rate of adverse events among 278 patients in 11 studies was 61.36% (95% CI 46.08-75.72, p< 0.01, I2:80%). Results are summarized in Figure 1.
Discussion: Our findings suggest that vedolizumab shows promise in treating chronic pouchitis, with clinical and endoscopic response rates of 61.36% and 57.92%, respectively. The comparison of disease remission rates for vedolizumab versus placebo or adalimumab plus infliximab suggests that vedolizumab may be superior to other biologic agents. The high adverse event rate of 61.36% highlights the necessity for careful monitoring. Overall, vedolizumab appears effective, but standardized research and further studies are essential to confirm its long-term efficacy and safety, enabling better management of chronic pouchitis.

Figure: Figure 1: A. Forest plot of the pooled rate of clinical response with vedolizumab. B. Forest plot of the pooled rate of endoscopic response with vedolizumab. C. Forest plot of the odd’s ratio of disease remission of vedolizumab compared to either placebo or infliximab plus adalimumab
Disclosures:
Nihal Khan indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Syed Hamaad Rahman indicated no relevant financial relationships.
Aimen Farooq indicated no relevant financial relationships.
Abu Hurairah indicated no relevant financial relationships.
Nihal I. Khan, MD1, Nouman Shafique, MD1, Syed Hamaad Rahman, DO2, Aimen Farooq, MD3, Abu Hurairah, MD1. P0965 - Efficacy and Safety of Vedolizumab in Chronic Pouchitis: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
