Sunday Poster Session
Category: IBD
P0969 - Sustained Efficacy and Safety After 5 Years of Continuous Ozanimod Treatment Independent of Clinical Remission Status: An Interim Analysis of the True North Open-Label Extension Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- DM
Dimpy Mehra, PharmD
Bristol Myers Squibb
Princeton, NJ
Presenting Author(s)
Raymond K. Cross, MD, MS, FACG1, Silvio Danese, MD, PhD2, Douglas C. Wolf, MD, FACG3, Katsuyoshi Matsuoka, MD4, Iris Dotan, MD5, Brigid S. Boland, MD6, Laura E. Raffals, MD7, Dimpy Mehra, PharmD8, Norma Ruiz Santiago, MD8, AnnKatrin Petersen, MD8, Manik Desai, PhD8, Hsiuanlin Wu, MS8, Dong Wang, PhD9, Mark T. Osterman, MD8, Anjali Jain, PhD8, James O. Lindsay, PhD10
1Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD; 2Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 3Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 4Toho University Sakura Medical Center, Sakura, Chiba, Japan; 5Rabin Medical Center, Tel Aviv University, Petah Tikva, Tel Aviv, Israel; 6University of California San Diego, La Jolla, CA; 7Mayo Clinic, Rochester, MN; 8Bristol Myers Squibb, Princeton, NJ; 9Bristol Myers Squibb K. K., Chiyoda City, Tokyo, Japan; 10Barts and the London School of Medicine and Dentistry, London, England, United Kingdom
Introduction: Ozanimod (OZA) was efficacious and well tolerated over 52 wk in patients (pts) with moderately to severely active ulcerative colitis (UC) in the phase 3 True North (TN) study (NCT02435992). Prior analyses of the ongoing TN open-label extension (OLE; NCT02531126) through OLE Week (W) 142 have demonstrated the efficacy and safety of continuous OZA up to ~4 y.
Methods: The efficacy and safety of OZA were assessed for an additional year in pts who completed OLE W190 (~5 y of continuous OZA) or discontinued the OLE by data cutoff (January 10, 2024). Pts on continuous OZA throughout TN who achieved clinical response (with or without also achieving clinical remission) at TN W52 and subsequently entered the OLE (referred to as W52 clinical responders) were included in this analysis. Subgroups of W52 clinical responders were also assessed; W52 clinical remitters achieved clinical response and remission and W52 clinical nonremitters achieved clinical response but not remission. Efficacy outcomes were evaluated through OLE W190. Treatment-emergent adverse events (TEAEs) were monitored during TN and the OLE through data cutoff.
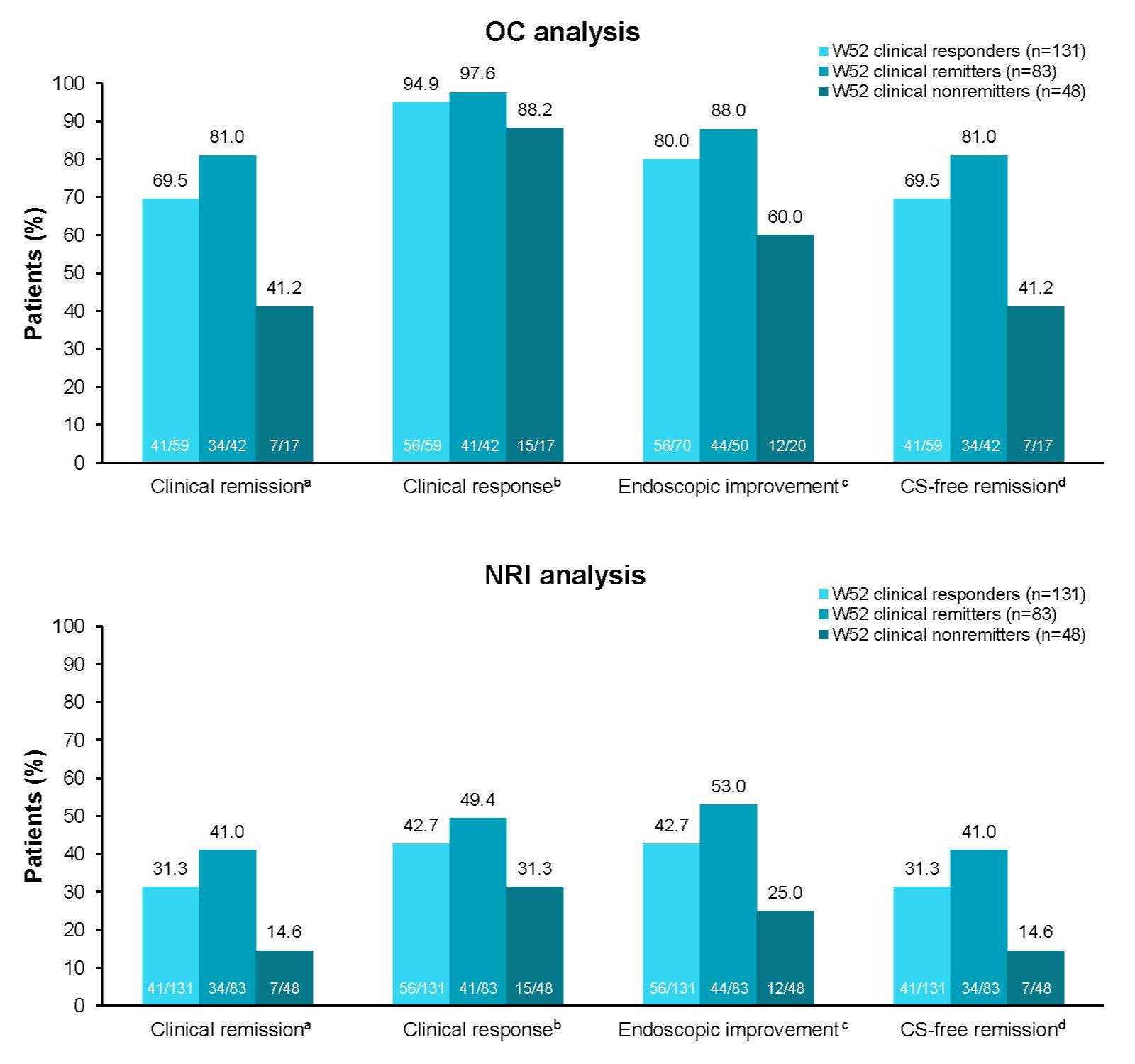
Results: Of the 131 W52 clinical responders who entered the OLE, 83 (63.4%) were also W52 clinical remitters. Symptomatic responses at OLE W190 were similar in W52 clinical remitters vs nonremitters (97.7% [43/44] vs 100.0% [19/19] in the observed case analysis and 51.8% [43/83] vs 39.6% [19/48] in the nonresponder imputation analysis). Rates of clinical remission, clinical response, endoscopic improvement, and corticosteroid-free remission at OLE W190 were higher in W52 clinical remitters vs nonremitters (Figure). Rates of TEAEs, serious TEAEs, TEAEs leading to discontinuation, and TEAEs of interest were generally similar across groups (Table). Rates of serious cardiac events were low. No serious hepatic events occurred.
Discussion: Symptomatic, mucosal, and corticosteroid-free clinical outcomes with continuous OZA treatment were sustained up to ~5 y, with the greatest benefit in pts who achieved clinical remission after 52 wk of OZA treatment. Long-term OZA treatment over ~5 y continues to be well tolerated with no new safety signals regardless of clinical remission status.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Raymond K. Cross, MD, MS, FACG1, Silvio Danese, MD, PhD2, Douglas C. Wolf, MD, FACG3, Katsuyoshi Matsuoka, MD4, Iris Dotan, MD5, Brigid S. Boland, MD6, Laura E. Raffals, MD7, Dimpy Mehra, PharmD8, Norma Ruiz Santiago, MD8, AnnKatrin Petersen, MD8, Manik Desai, PhD8, Hsiuanlin Wu, MS8, Dong Wang, PhD9, Mark T. Osterman, MD8, Anjali Jain, PhD8, James O. Lindsay, PhD10. P0969 - Sustained Efficacy and Safety After 5 Years of Continuous Ozanimod Treatment Independent of Clinical Remission Status: An Interim Analysis of the True North Open-Label Extension Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD; 2Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 3Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 4Toho University Sakura Medical Center, Sakura, Chiba, Japan; 5Rabin Medical Center, Tel Aviv University, Petah Tikva, Tel Aviv, Israel; 6University of California San Diego, La Jolla, CA; 7Mayo Clinic, Rochester, MN; 8Bristol Myers Squibb, Princeton, NJ; 9Bristol Myers Squibb K. K., Chiyoda City, Tokyo, Japan; 10Barts and the London School of Medicine and Dentistry, London, England, United Kingdom
Introduction: Ozanimod (OZA) was efficacious and well tolerated over 52 wk in patients (pts) with moderately to severely active ulcerative colitis (UC) in the phase 3 True North (TN) study (NCT02435992). Prior analyses of the ongoing TN open-label extension (OLE; NCT02531126) through OLE Week (W) 142 have demonstrated the efficacy and safety of continuous OZA up to ~4 y.
Methods: The efficacy and safety of OZA were assessed for an additional year in pts who completed OLE W190 (~5 y of continuous OZA) or discontinued the OLE by data cutoff (January 10, 2024). Pts on continuous OZA throughout TN who achieved clinical response (with or without also achieving clinical remission) at TN W52 and subsequently entered the OLE (referred to as W52 clinical responders) were included in this analysis. Subgroups of W52 clinical responders were also assessed; W52 clinical remitters achieved clinical response and remission and W52 clinical nonremitters achieved clinical response but not remission. Efficacy outcomes were evaluated through OLE W190. Treatment-emergent adverse events (TEAEs) were monitored during TN and the OLE through data cutoff.
Results: Of the 131 W52 clinical responders who entered the OLE, 83 (63.4%) were also W52 clinical remitters. Symptomatic responses at OLE W190 were similar in W52 clinical remitters vs nonremitters (97.7% [43/44] vs 100.0% [19/19] in the observed case analysis and 51.8% [43/83] vs 39.6% [19/48] in the nonresponder imputation analysis). Rates of clinical remission, clinical response, endoscopic improvement, and corticosteroid-free remission at OLE W190 were higher in W52 clinical remitters vs nonremitters (Figure). Rates of TEAEs, serious TEAEs, TEAEs leading to discontinuation, and TEAEs of interest were generally similar across groups (Table). Rates of serious cardiac events were low. No serious hepatic events occurred.
Discussion: Symptomatic, mucosal, and corticosteroid-free clinical outcomes with continuous OZA treatment were sustained up to ~5 y, with the greatest benefit in pts who achieved clinical remission after 52 wk of OZA treatment. Long-term OZA treatment over ~5 y continues to be well tolerated with no new safety signals regardless of clinical remission status.

Figure: Figure. OZA efficacy at OLE W190 in TN W52 clinical responders, remitters, and nonremitters.
aRBS = 0, SFS ≤1 (and ≥1-point decrease from baseline SFS), and MES ≤1. b≥2-point and ≥35% decrease from baseline in the 3-component Mayo score (sum of RBS, SFS, and MES) and ≥1-point decrease in RBS or absolute RBS of ≤1. cMES ≤1. dClinical remission while off CS for ≥12 wk.
CS, corticosteroid; MES, Mayo endoscopy subscore; NRI, nonresponder imputation; OC, observed case; OLE, open-label extension; OZA, ozanimod; RBS, rectal bleeding subscore; SFS, stool frequency subscore; TN, True North; W, Week.
aRBS = 0, SFS ≤1 (and ≥1-point decrease from baseline SFS), and MES ≤1. b≥2-point and ≥35% decrease from baseline in the 3-component Mayo score (sum of RBS, SFS, and MES) and ≥1-point decrease in RBS or absolute RBS of ≤1. cMES ≤1. dClinical remission while off CS for ≥12 wk.
CS, corticosteroid; MES, Mayo endoscopy subscore; NRI, nonresponder imputation; OC, observed case; OLE, open-label extension; OZA, ozanimod; RBS, rectal bleeding subscore; SFS, stool frequency subscore; TN, True North; W, Week.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Raymond Cross: AbbVie – Consultant. Adiso – Consultant. Bristol Myers Squibb – Consultant. CorEvitas Registry – Scientific co-director. Fresenius Kabi – Consultant. Fzata – Consultant. IBD Education Group – Executive committee member. Janssen – Consultant, Grant/Research Support. Magellan Health – Consultant. Option Care Health – Consultant. Pfizer – Consultant. Pharmacosmos – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant. Sebela – Consultant. Takeda – Consultant.
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Applied Molecular Transport – Consultant. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion Healthcare – Consultant. Dr Falk Pharma – Consultant. Eli Lilly and Company – Consultant. Enthera – Consultant. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant. Inotrem – Consultant. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant. Morphic – Consultant. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Speakers Bureau. Teladoc Health – Consultant. TiGenix – Consultant. UCB Inc. – Consultant. Vial – Consultant. Vifor – Consultant.
Douglas C. Wolf: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Amgen – Grant/Research Support. Arena/Pfizer – Consultant, Grant/Research Support. Celgene/Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant. Genentech – Grant/Research Support. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Katsuyoshi Matsuoka: AbbVie – Grant/Research Support, Speakers Bureau. EA Pharma – Speakers Bureau. Eli Lilly – Speakers Bureau. Gilead – Speakers Bureau. Janssen – Speakers Bureau. JIMRO – Grant/Research Support. Kissei – Speakers Bureau. Mitsubishi Tanabe – Speakers Bureau. Mochida – Grant/Research Support, Speakers Bureau. Nippon Kayaku – Grant/Research Support. Pfizer – Speakers Bureau. Takeda – Speakers Bureau. Zeria – Grant/Research Support.
Iris Dotan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Altman – Advisory Committee/Board Member, Consultant, Speakers Bureau. Athos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene/Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gutreat and Harp Diagnostics – shareholder. Iterative Scopes – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Prometheus – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche/Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sangamo – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sublimity – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Brigid S. Boland: Bristol Myers Squibb – Consultant. Gilead – Grant/Research Support. Merck – Grant/Research Support. Pfizer – Consultant.
Laura E. Raffals: Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Fresenius Kabi USA – Advisory Committee/Board Member, Consultant. Geneoscopy – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Roivant Sciences – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant.
Dimpy Mehra: Bristol Myers Squibb – Employee.
Norma Ruiz Santiago: Bristol Myers Squibb – Employee.
AnnKatrin Petersen: Bristol Myers Squibb – Employee.
Manik Desai: Bristol Myers Squibb – Employee.
Hsiuanlin Wu: Bristol Myers Squibb – Employee.
Dong Wang: Bristol Myers Squibb – Employee.
Mark T. Osterman: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
James O. Lindsay: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Engitix – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Napp – Consultant, Speakers Bureau. Orchard Therapeutics – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Raymond K. Cross, MD, MS, FACG1, Silvio Danese, MD, PhD2, Douglas C. Wolf, MD, FACG3, Katsuyoshi Matsuoka, MD4, Iris Dotan, MD5, Brigid S. Boland, MD6, Laura E. Raffals, MD7, Dimpy Mehra, PharmD8, Norma Ruiz Santiago, MD8, AnnKatrin Petersen, MD8, Manik Desai, PhD8, Hsiuanlin Wu, MS8, Dong Wang, PhD9, Mark T. Osterman, MD8, Anjali Jain, PhD8, James O. Lindsay, PhD10. P0969 - Sustained Efficacy and Safety After 5 Years of Continuous Ozanimod Treatment Independent of Clinical Remission Status: An Interim Analysis of the True North Open-Label Extension Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
