Sunday Poster Session
Category: IBD
P0970 - Impact of Baseline Albumin Levels on Ozanimod Efficacy: A Post Hoc Analysis of the Phase 3 True North Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- DM
Dimpy Mehra, PharmD
Bristol Myers Squibb
Princeton, NJ
Presenting Author(s)
Raymond K. Cross, MD, MS, FACG1, Edward L. Barnes, MD, MPH2, Adam S. Cheifetz, MD3, Shomron Ben-Horin, MD4, Xavier Roblin, MD5, Andres Yarur, MD6, Zhaohui Liu, PhD7, Dimpy Mehra, PharmD7, Sarah Harris, PhD8, Mark T. Osterman, MD7, Garrett Lawlor, MD7, Anjali Jain, PhD7, Parambir S. Dulai, MD9
1Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD; 2School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC; 3Beth Israel Deaconess Medical Center, Boston, MA; 4Sheba Medical Center, Ramat Gan, Tel Aviv, Israel; 5University of St. Etienne, Saint-Étienne, Ile-de-France, France; 6Cedars-Sinai Medical Center, Los Angeles, CA; 7Bristol Myers Squibb, Princeton, NJ; 8Bristol Myers Squibb, San Diego, CA; 9Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Ozanimod (OZA), a selective sphingosine 1-phosphate 1 and 5 receptor modulator, is an efficacious and well-tolerated therapy for patients (pts) with moderately to severely active ulcerative colitis (UC). Serum albumin is inversely associated with the drug clearance of biologic therapies in inflammatory bowel disease (IBD), thus impacting their efficacy.
Methods: This post hoc analysis assessed the relationship between baseline albumin levels and OZA response in the phase 3 True North study (NCT02435992). Pts who were clinical responders after 10 weeks of OZA treatment were rerandomized to OZA or placebo for an additional 42 weeks through Week (W) 52. Baseline albumin levels were assessed using quartile analysis, and the percentage of pts who achieved clinical response at W10 in each quartile was determined. Multivariable logistic regression using stepwise selection was used to identify baseline clinical and biomarker variables that impacted clinical response to OZA at W10.
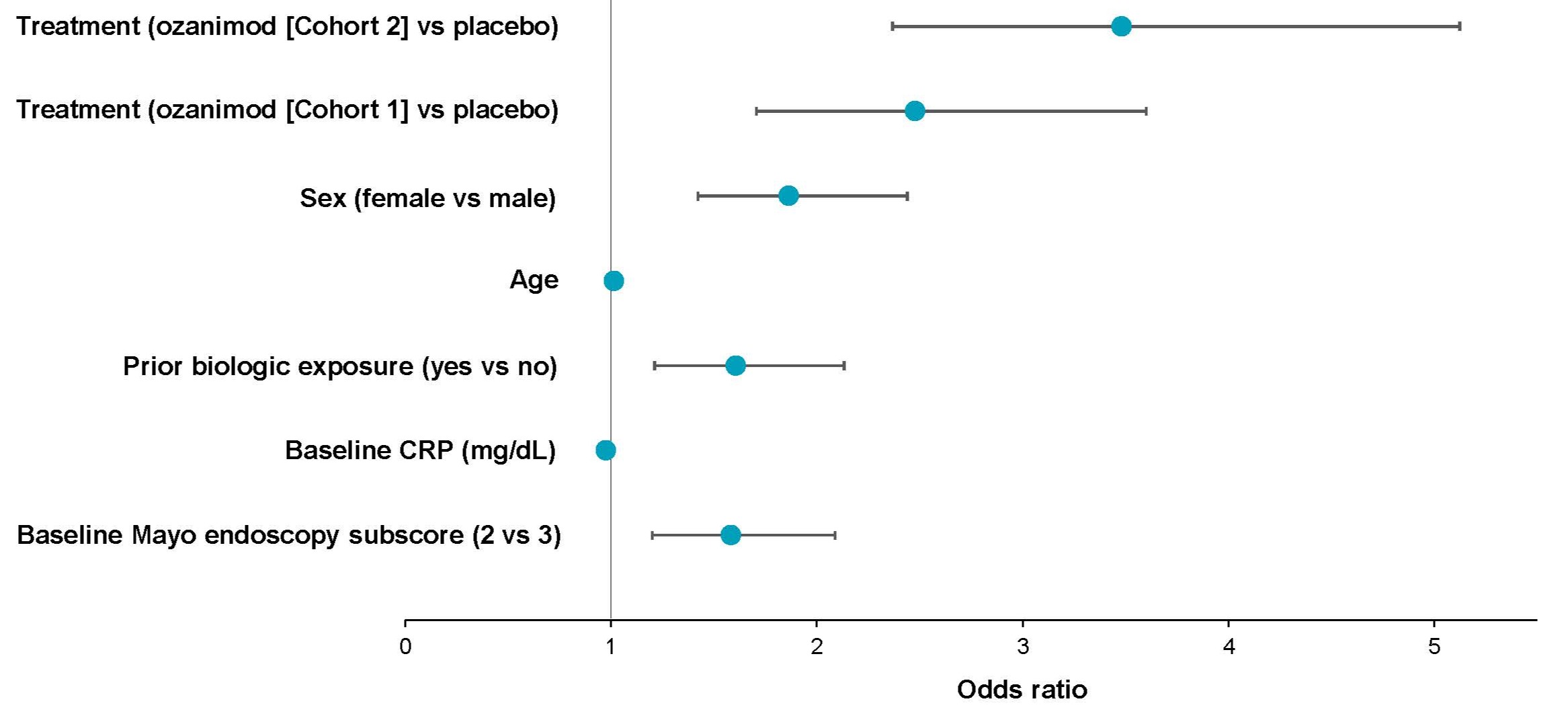
Results: As reported previously, 47.8% (205/429) and 52.6% (193/367) of OZA-treated pts in Cohort 1 and Cohort 2, respectively, achieved clinical response at W10. Mean baseline albumin levels were similar in W10 clinical responders vs nonresponders (4.21 vs 4.17 g/dL in OZA Cohort 1; 4.33 vs 4.23 g/dL in OZA Cohort 2). In a quartile analysis of baseline albumin levels, the percentages of W10 clinical responders among OZA-treated pts (Cohorts 1 and 2 combined) slightly increased from 42.2% in Quartile 1 (1.7–4.0 g/dL) to 56.4% in Quartile 4 (4.5–5.6 g/dL). However, in the multivariable logistic regression model, baseline albumin levels did not influence clinical response to OZA at W10, which was primarily driven by sex, baseline endoscopic disease severity, and prior biologic exposure status (Figure).
Discussion: While low baseline albumin levels have been associated with lower efficacy of biologics in IBD, this link was not observed with OZA. Baseline serum albumin levels were similar regardless of clinical response at the end of induction with OZA treatment. Furthermore, prior biologic exposure and baseline endoscopic disease severity, but not baseline albumin levels, were significant predictors of OZA clinical response at W10. These results suggest that albumin levels do not impact OZA efficacy in pts with UC.
Disclosures:
Raymond K. Cross, MD, MS, FACG1, Edward L. Barnes, MD, MPH2, Adam S. Cheifetz, MD3, Shomron Ben-Horin, MD4, Xavier Roblin, MD5, Andres Yarur, MD6, Zhaohui Liu, PhD7, Dimpy Mehra, PharmD7, Sarah Harris, PhD8, Mark T. Osterman, MD7, Garrett Lawlor, MD7, Anjali Jain, PhD7, Parambir S. Dulai, MD9. P0970 - Impact of Baseline Albumin Levels on Ozanimod Efficacy: A Post Hoc Analysis of the Phase 3 True North Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Melissa L. Posner Institute for Digestive Health & Liver Disease at Mercy Medical Center, Baltimore, MD; 2School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC; 3Beth Israel Deaconess Medical Center, Boston, MA; 4Sheba Medical Center, Ramat Gan, Tel Aviv, Israel; 5University of St. Etienne, Saint-Étienne, Ile-de-France, France; 6Cedars-Sinai Medical Center, Los Angeles, CA; 7Bristol Myers Squibb, Princeton, NJ; 8Bristol Myers Squibb, San Diego, CA; 9Feinberg School of Medicine, Northwestern University, Chicago, IL
Introduction: Ozanimod (OZA), a selective sphingosine 1-phosphate 1 and 5 receptor modulator, is an efficacious and well-tolerated therapy for patients (pts) with moderately to severely active ulcerative colitis (UC). Serum albumin is inversely associated with the drug clearance of biologic therapies in inflammatory bowel disease (IBD), thus impacting their efficacy.
Methods: This post hoc analysis assessed the relationship between baseline albumin levels and OZA response in the phase 3 True North study (NCT02435992). Pts who were clinical responders after 10 weeks of OZA treatment were rerandomized to OZA or placebo for an additional 42 weeks through Week (W) 52. Baseline albumin levels were assessed using quartile analysis, and the percentage of pts who achieved clinical response at W10 in each quartile was determined. Multivariable logistic regression using stepwise selection was used to identify baseline clinical and biomarker variables that impacted clinical response to OZA at W10.
Results: As reported previously, 47.8% (205/429) and 52.6% (193/367) of OZA-treated pts in Cohort 1 and Cohort 2, respectively, achieved clinical response at W10. Mean baseline albumin levels were similar in W10 clinical responders vs nonresponders (4.21 vs 4.17 g/dL in OZA Cohort 1; 4.33 vs 4.23 g/dL in OZA Cohort 2). In a quartile analysis of baseline albumin levels, the percentages of W10 clinical responders among OZA-treated pts (Cohorts 1 and 2 combined) slightly increased from 42.2% in Quartile 1 (1.7–4.0 g/dL) to 56.4% in Quartile 4 (4.5–5.6 g/dL). However, in the multivariable logistic regression model, baseline albumin levels did not influence clinical response to OZA at W10, which was primarily driven by sex, baseline endoscopic disease severity, and prior biologic exposure status (Figure).
Discussion: While low baseline albumin levels have been associated with lower efficacy of biologics in IBD, this link was not observed with OZA. Baseline serum albumin levels were similar regardless of clinical response at the end of induction with OZA treatment. Furthermore, prior biologic exposure and baseline endoscopic disease severity, but not baseline albumin levels, were significant predictors of OZA clinical response at W10. These results suggest that albumin levels do not impact OZA efficacy in pts with UC.

Figure: Figure. Predictors of OZA clinical response at W10. Multivariable logistic regression using stepwise selection was used to predict clinical response to OZA at W10. Fifteen variables were included in the model: baseline albumin level, CS use at screening, prior biologic exposure, age, sex, extent of disease within the past 2 years, moderate UC status at baseline, prior CS exposure, prior oral 5-ASA exposure, prior immunomodulator exposure, prior CS and 5-ASA exposure, baseline Mayo endoscopy subscore, baseline total Mayo score, baseline partial Mayo score, and baseline CRP (mg/dL). Error bars represent 95% CIs. 5-ASA, 5-aminosalicylate; CRP, C-reactive protein; CS, corticosteroid; OZA, ozanimod; UC, ulcerative colitis; W, Week.
Disclosures:
Raymond K. Cross: AbbVie – Consultant. Adiso – Consultant. Bristol Myers Squibb – Consultant. CorEvitas Registry – scientific co-director. Fresenius Kabi – Consultant. Fzata – Consultant. IBD Education Group – executive committee member. Janssen – Consultant, Grant/Research Support. Magellan Health – Consultant. Option Care Health – Consultant. Pfizer – Consultant. Pharmacosmos – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant. Sebela – Consultant. Takeda – Consultant.
Edward L. Barnes: AbbVie – Consultant. Boomerang – Consultant. Bristol Myers Squibb – Consultant. Direct Biologics – Consultant. Eli Lilly – Consultant. Pfizer – Consultant. Target RWE – Consultant.
Adam S. Cheifetz: AbbVie – Consultant, Speakers Bureau. Adiso – Consultant. AegirBio – Consultant. Artizan – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Clario – Consultant. Eli Lilly – Consultant. Food is Good – Consultant. Fresenius Kabi – Consultant. Fzata – Consultant. Janssen – Consultant. Pfizer – Consultant. Procise – Consultant. Prometheus – Consultant. Samsung – Consultant. Spherix – Consultant.
Shomron Ben-Horin: AbbVie – Advisory Committee/Board Member, Consultant, Stock Options. Alma Therapeutics – Stock Options. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celltrion – Advisory Committee/Board Member, Consultant, Stock Options. Eli Lilly – Advisory Committee/Board Member, Consultant. Evinature – Stock Options. Ferring – Advisory Committee/Board Member, Consultant. Galmed – Advisory Committee/Board Member, Consultant, Stock Options. Gilead – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Stock Options. Medial EarlySign – Advisory Committee/Board Member, Consultant. Medtronic – Grant/Research Support. NeoPharm – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support. Predicta Med – Advisory Committee/Board Member, Consultant, Stock Options. Roche – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support.
Xavier Roblin: 4SC – personal fees. AbbVie – personal fees. Aesca – personal fees. Algernon – personal fees. AM Pharma – personal fees. Amgen – personal fees. AMT – personal fees. AOP Orphan – personal fees. Aptalis – personal fees. Arena Pharmaceuticals – personal fees. Astellas – personal fees. AstraZeneca – personal fees. Avaxia – personal fees. Bioclinica – personal fees. Biogen IDEC – personal fees. Boehringer Ingelheim – personal fees. Bristol Myers Squibb – personal fees. Celgene – personal fees. Cellerix – personal fees. Celltrion – personal fees. Centocor – personal fees. Chemocentryx – personal fees. Covance – personal fees. Danone Austria – personal fees. DSM – personal fees. Elan – personal fees. Eli Lilly – personal fees. Ernst & Young – personal fees. Falk – personal fees. Ferring – personal fees. Galapagos – personal fees. Gatehouse Bio Inc. – personal fees. Genentech – personal fees. Gilead – personal fees. Grünenthal – personal fees. ICON – personal fees. Immundiagnostik – personal fees. InDex Pharmaceuticals – personal fees. Inova – personal fees. Intrinsic Imaging – personal fees. J&J – personal fees. Janssen – personal fees. Kyowa Hakko Kirin Pharma – personal fees. Lipid Therapeutics – personal fees. LivaNova – personal fees. Mallinckrodt – personal fees. Medahead – personal fees. MedImmune – personal fees. Millenium – personal fees. Mitsubishi Tanabe Pharma Corporation – personal fees. MSD – personal fees. Nash Pharmaceuticals – personal fees. Nestle – personal fees. Nippon Kayaku – personal fees. Novartis – personal fees. Ocera – personal fees. OMass – personal fees. Otsuka – personal fees. Parexel – personal fees. PDL – personal fees. Periconsulting – personal fees. Pfizer – personal fees. Pharmacosmos – personal fees. Philip Morris Institute – personal fees. PLS Education – personal fees. Procter & Gamble – personal fees. Prometheus – personal fees. Protagonist – personal fees. Provention – personal fees. Quell Therapeutics – personal fees. Robarts Clinical Trial – personal fees. Roland Berger GmBH – personal fees. Sandoz – personal fees. Schering-Plough – personal fees. Second Genome – personal fees. Seres Therapeutics – personal fees. Setpointmedical – personal fees. Shire – personal fees. Sigmoid – personal fees. Sublimity – personal fees. Takeda – personal fees. Therakos – personal fees. Theravance – personal fees. TiGenix – personal fees. Tillots – personal fees. UCB – personal fees. Vifor – personal fees. Yakult – personal fees. Zeeland – personal fees. Zyngenia – personal fees.
Andres Yarur: AbbVie – Consultant, served on clinical trial steering committee. Abivax – Advisory Committee/Board Member, Consultant. Arena – Consultant, served on clinical trial steering committee. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, served on clinical trial steering committee. Celltrion – Consultant, served on clinical trial steering committee. Johnson and Johnson – Advisory Committee/Board Member, Consultant. Pfizer – Consultant, served on clinical trial steering committee. Takeda – Consultant, served on clinical trial steering committee.
Zhaohui Liu: Bristol Myers Squibb – Employee.
Dimpy Mehra: Bristol Myers Squibb – Employee.
Sarah Harris: Bristol Myers Squibb – Employee.
Mark T. Osterman: Bristol Myers Squibb – Employee.
Garrett Lawlor: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
Parambir Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Bristol Meyer Squibb – Consultant. Digbi Health – Royalties. Digbi Health – Stock Options. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Licensing royalties. Takeda – Consultant, Grant/Research Support.
Raymond K. Cross, MD, MS, FACG1, Edward L. Barnes, MD, MPH2, Adam S. Cheifetz, MD3, Shomron Ben-Horin, MD4, Xavier Roblin, MD5, Andres Yarur, MD6, Zhaohui Liu, PhD7, Dimpy Mehra, PharmD7, Sarah Harris, PhD8, Mark T. Osterman, MD7, Garrett Lawlor, MD7, Anjali Jain, PhD7, Parambir S. Dulai, MD9. P0970 - Impact of Baseline Albumin Levels on Ozanimod Efficacy: A Post Hoc Analysis of the Phase 3 True North Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
