Sunday Poster Session
Category: IBD
P0977 - Breakthrough Cutaneous Crohn's Disease Healed with Risankizumab
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Kristen Brennan, DO
Medical College of Wisconsin
Milwaukee, WI
Presenting Author(s)
Kristen Brennan, DO, Daniel J.. Stein, MD, Gretchen Roth, MD
Medical College of Wisconsin, Milwaukee, WI
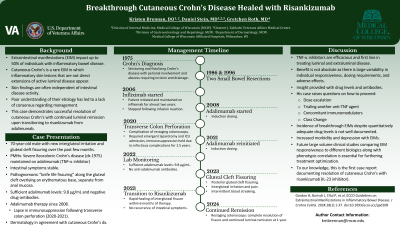
Introduction: Metastatic Crohn’s disease is a rare extraintestinal manifestation (EIM) in which inflammatory skin lesions that are not direct extensions of active luminal disease appear, often independent of intestinal disease activity. The limited nature of these lesions and poor understanding of their etiology has led to a lack of consensus regarding management. This case demonstrates successful resolution of cutaneous Crohn’s with continued luminal remission upon transitioning to riskanizumab from adalimumab.
Case Description/Methods: A 75-year-old male with history significant for severe Ileocolonic Crohn’s disease, maintained on adalimumab, presented with complaints of new intergluteal irritation and gluteal cleft fissuring over the past few months. On exam, he was found to have pathognomonic “knife-like fissuring” along the gluteal cleft overlying an erythematous base, separate from the anal mucosa.
The patient’s 2020 re-staging colonoscopy was complicated by a transverse colon perforation requiring emergent laparotomy and ICU admission. His immunosuppression was held during this time due to infectious complications during his recovery over the next year and a half. Given his lapse in biologic therapy, quantitative drug levels and antibody testing were ordered following re-induction with adalimumab which demonstrated therapeutic levels at 9.8 ug/mL without antibodies.
Progression and development of posterior gluteal cleft fissuring was noted during office visit on 4/19/2023. He was evaluated by dermatology who agreed with the diagnosis of Cutaneous Crohn’s and jointly monitored his disease. Despite adequate levels and stable intestinal symptoms, the patient was transitioned to riskanizumab. His intergluteal fissure began healing within six months of therapy. Restaging colonoscopy one year later demonstrated complete resolution of his fissure and continued luminal remission on riskanizumab.
Discussion: The incidence of breakthrough EIMs despite quantitatively adequate drug levels is not well documented. The FDA has approved risankizumab, an Interleukin 23A inhibitor traditionally used in patients battling plaque psoriasis and psoriatic arthritis for individuals with moderate to severe Crohn’s disease. IL-23 inhibitors are a promising therapy option for those plagued with cutaneous Crohn’s disease. To our knowledge, this is the first case documenting resolution of cutaneous Crohn’s disease with risankizumab.
Disclosures:
Kristen Brennan, DO, Daniel J.. Stein, MD, Gretchen Roth, MD. P0977 - Breakthrough Cutaneous Crohn's Disease Healed with Risankizumab, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Medical College of Wisconsin, Milwaukee, WI
Introduction: Metastatic Crohn’s disease is a rare extraintestinal manifestation (EIM) in which inflammatory skin lesions that are not direct extensions of active luminal disease appear, often independent of intestinal disease activity. The limited nature of these lesions and poor understanding of their etiology has led to a lack of consensus regarding management. This case demonstrates successful resolution of cutaneous Crohn’s with continued luminal remission upon transitioning to riskanizumab from adalimumab.
Case Description/Methods: A 75-year-old male with history significant for severe Ileocolonic Crohn’s disease, maintained on adalimumab, presented with complaints of new intergluteal irritation and gluteal cleft fissuring over the past few months. On exam, he was found to have pathognomonic “knife-like fissuring” along the gluteal cleft overlying an erythematous base, separate from the anal mucosa.
The patient’s 2020 re-staging colonoscopy was complicated by a transverse colon perforation requiring emergent laparotomy and ICU admission. His immunosuppression was held during this time due to infectious complications during his recovery over the next year and a half. Given his lapse in biologic therapy, quantitative drug levels and antibody testing were ordered following re-induction with adalimumab which demonstrated therapeutic levels at 9.8 ug/mL without antibodies.
Progression and development of posterior gluteal cleft fissuring was noted during office visit on 4/19/2023. He was evaluated by dermatology who agreed with the diagnosis of Cutaneous Crohn’s and jointly monitored his disease. Despite adequate levels and stable intestinal symptoms, the patient was transitioned to riskanizumab. His intergluteal fissure began healing within six months of therapy. Restaging colonoscopy one year later demonstrated complete resolution of his fissure and continued luminal remission on riskanizumab.
Discussion: The incidence of breakthrough EIMs despite quantitatively adequate drug levels is not well documented. The FDA has approved risankizumab, an Interleukin 23A inhibitor traditionally used in patients battling plaque psoriasis and psoriatic arthritis for individuals with moderate to severe Crohn’s disease. IL-23 inhibitors are a promising therapy option for those plagued with cutaneous Crohn’s disease. To our knowledge, this is the first case documenting resolution of cutaneous Crohn’s disease with risankizumab.
Disclosures:
Kristen Brennan indicated no relevant financial relationships.
Daniel Stein: Abbvie – Consultant, Speakers Bureau. BMS – Consultant, Speakers Bureau. Janssen – Speakers Bureau. Lilly – Speakers Bureau. Pfizer – Speakers Bureau. Takeda – Speakers Bureau.
Gretchen Roth: Incyte – Grant/Research Support. Lilly – Grant/Research Support. Moonlake – Grant/Research Support.
Kristen Brennan, DO, Daniel J.. Stein, MD, Gretchen Roth, MD. P0977 - Breakthrough Cutaneous Crohn's Disease Healed with Risankizumab, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
