Sunday Poster Session
Category: Interventional Endoscopy
P1054 - Radiofrequency Vapor Ablation is Safe and Well-Tolerated for Duodenal Mucosal Ablation in the Treatment of Type 2 Diabetes: Six Month Results From the First-in-Human Dose Finding Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Benjamin Norton, MRCP
Cleveland Clinic
London, England, United Kingdom
Presenting Author(s)
Award: Presidential Poster Award
Benjamin Norton, MRCP1, Apostolis Papaefthymiou, MD1, Andrea Telese, MD1, Leonardo Rodriguez Grunert, 2, Pablo Hoebel, MD2, Paulina Vignoloa, MD2, Charles Murray, MD1, Kenneth Chang, MD3, C Roberto Simon-Linares, MD4, Rehan Haidry, MD1
1Cleveland Clinic, London, England, United Kingdom; 2Clinica Colonial, Santiago, Region Metropolitana, Chile; 3University of California Irvine, Irvine, CA; 4Cleveland Clinic, Cleveland, OH
Introduction: Duodenal mucosal ablation (DA) is an emerging endoscopic treatment for type 2 diabetes (T2D). The FDA-cleared Radiofrequency Vapor Ablation (RFVA) system (AquaMedical Inc., Pleasanton, CA) is a single-use, 10.5Fr through-the-endoscope, circumferential ablation catheter used for DA. RF energy converts saline into heated vapor at the catheter tip which is released into the duodenum under direct endoscopic visualization. In this first-in-human pilot dosimetry study, we assessed the safety, tolerability, and initial efficacy of RFVA for DA in patients with T2D (NCT05887635).
Methods: This was a prospective, single-center trial enrolling 27 patients with uncontrolled T2D (glycated hemoglobin (HbA1c) 7.5-10 %) despite ≥1 oral antidiabetic drug. After a 4-week stable glycemic run-in, DA was performed under anesthesia followed by a modified diet for 14 days. Technical success was defined as ≥9 cm of post-ampullary duodenal ablation (Fig. 1). Stepwise dose titrations were performed as follows: safety dose (180J 1x; n=2), first treatment dose (180J 2x; n=11), second treatment dose (200J 2x; n=14). The primary outcomes were safety (number of serious adverse events (SAEs)), tolerability (mean visual analog scale (VAS) score), and efficacy at various RFVA doses (change in HbA1c at 12-/24-weeks).
Results: Twenty-seven patients with a mean (SD) T2D duration of 6 years (2.6) underwent DA. The mean age was 54 years (6.6), the baseline BMI 30.3 kg/m2 (4.2), and 48.2% were men. The baseline HbA1c was 8.5% (0.7) and fasting glucose 160.1 mg/dL (46.8). Technical success was 100%. In the final dose cohort, the mean procedure time was 43.6 minutes (9.2), catheter time 30.6 minutes (7.0), number of ablations 17.6 (2.7), and ablation length 14.3 cm (1.8). There were no SAEs or hypoglycemia. Patients were discharged the following day per-protocol, except two same-day. Repeat endoscopy and duodenal biopsies at four weeks were normal (n=27). RFVA was well-tolerated with a max mean VAS pain score of 1.4 (2.1) on day 2. We observed a significant reduction in HbA1c, fasting and post-prandial fingerstick glucose at 12- and 24-weeks in the 2x treatment cohorts (n=25; table 1), with no difference between doses at 12- or 24-weeks (p=NS; all comparisons). At 3-months, weight change correlated with HbA1c (r=.46; p=.02), but not 6-months (r=.05; p=.82).
Discussion: DA using RFVA is safe, well tolerated, and easy to perform for the management of poorly controlled T2D with excellent early efficacy.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Benjamin Norton, MRCP1, Apostolis Papaefthymiou, MD1, Andrea Telese, MD1, Leonardo Rodriguez Grunert, 2, Pablo Hoebel, MD2, Paulina Vignoloa, MD2, Charles Murray, MD1, Kenneth Chang, MD3, C Roberto Simon-Linares, MD4, Rehan Haidry, MD1. P1054 - Radiofrequency Vapor Ablation is Safe and Well-Tolerated for Duodenal Mucosal Ablation in the Treatment of Type 2 Diabetes: Six Month Results From the First-in-Human Dose Finding Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Benjamin Norton, MRCP1, Apostolis Papaefthymiou, MD1, Andrea Telese, MD1, Leonardo Rodriguez Grunert, 2, Pablo Hoebel, MD2, Paulina Vignoloa, MD2, Charles Murray, MD1, Kenneth Chang, MD3, C Roberto Simon-Linares, MD4, Rehan Haidry, MD1
1Cleveland Clinic, London, England, United Kingdom; 2Clinica Colonial, Santiago, Region Metropolitana, Chile; 3University of California Irvine, Irvine, CA; 4Cleveland Clinic, Cleveland, OH
Introduction: Duodenal mucosal ablation (DA) is an emerging endoscopic treatment for type 2 diabetes (T2D). The FDA-cleared Radiofrequency Vapor Ablation (RFVA) system (AquaMedical Inc., Pleasanton, CA) is a single-use, 10.5Fr through-the-endoscope, circumferential ablation catheter used for DA. RF energy converts saline into heated vapor at the catheter tip which is released into the duodenum under direct endoscopic visualization. In this first-in-human pilot dosimetry study, we assessed the safety, tolerability, and initial efficacy of RFVA for DA in patients with T2D (NCT05887635).
Methods: This was a prospective, single-center trial enrolling 27 patients with uncontrolled T2D (glycated hemoglobin (HbA1c) 7.5-10 %) despite ≥1 oral antidiabetic drug. After a 4-week stable glycemic run-in, DA was performed under anesthesia followed by a modified diet for 14 days. Technical success was defined as ≥9 cm of post-ampullary duodenal ablation (Fig. 1). Stepwise dose titrations were performed as follows: safety dose (180J 1x; n=2), first treatment dose (180J 2x; n=11), second treatment dose (200J 2x; n=14). The primary outcomes were safety (number of serious adverse events (SAEs)), tolerability (mean visual analog scale (VAS) score), and efficacy at various RFVA doses (change in HbA1c at 12-/24-weeks).
Results: Twenty-seven patients with a mean (SD) T2D duration of 6 years (2.6) underwent DA. The mean age was 54 years (6.6), the baseline BMI 30.3 kg/m2 (4.2), and 48.2% were men. The baseline HbA1c was 8.5% (0.7) and fasting glucose 160.1 mg/dL (46.8). Technical success was 100%. In the final dose cohort, the mean procedure time was 43.6 minutes (9.2), catheter time 30.6 minutes (7.0), number of ablations 17.6 (2.7), and ablation length 14.3 cm (1.8). There were no SAEs or hypoglycemia. Patients were discharged the following day per-protocol, except two same-day. Repeat endoscopy and duodenal biopsies at four weeks were normal (n=27). RFVA was well-tolerated with a max mean VAS pain score of 1.4 (2.1) on day 2. We observed a significant reduction in HbA1c, fasting and post-prandial fingerstick glucose at 12- and 24-weeks in the 2x treatment cohorts (n=25; table 1), with no difference between doses at 12- or 24-weeks (p=NS; all comparisons). At 3-months, weight change correlated with HbA1c (r=.46; p=.02), but not 6-months (r=.05; p=.82).
Discussion: DA using RFVA is safe, well tolerated, and easy to perform for the management of poorly controlled T2D with excellent early efficacy.

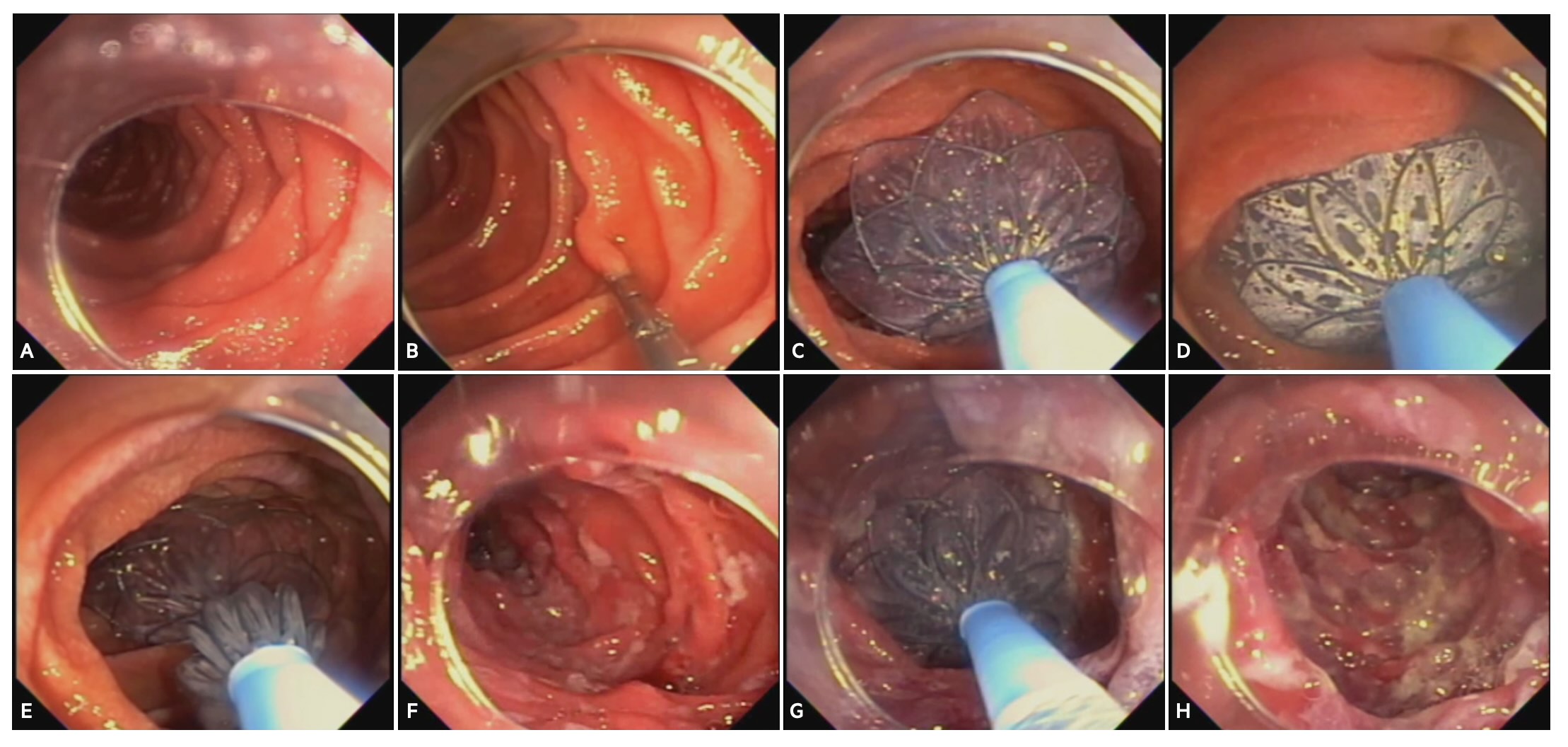
Figure: Figure 1 – Procedural steps of duodenal mucosal ablation using radiofrequency vapor ablation (RFVA)
A) Initial inspection and washing of the duodenum with 2% N-acetylcysteine. B) Papilla is identified and a clip is placed on the contralateral wall marking the proximal boundary. C) RFVA catheter is inserted through-the-endoscope followed by deployment and positioning of two 30 mm nitinol discs. D) Vapor ablation is delivered to the treatment zone. E) Discs are withdrawn and repositioned to the next treatment zone in a proximal-to-distal direction aiming for ≥9 cm of immediate post-ampullary duodenum. F) After the first run ablations, the treated duodenal segment is observed for any complications. G) A second run of ablations are completed by repeating steps C-E. H) A final review of the ablated segments after double application.
A) Initial inspection and washing of the duodenum with 2% N-acetylcysteine. B) Papilla is identified and a clip is placed on the contralateral wall marking the proximal boundary. C) RFVA catheter is inserted through-the-endoscope followed by deployment and positioning of two 30 mm nitinol discs. D) Vapor ablation is delivered to the treatment zone. E) Discs are withdrawn and repositioned to the next treatment zone in a proximal-to-distal direction aiming for ≥9 cm of immediate post-ampullary duodenum. F) After the first run ablations, the treated duodenal segment is observed for any complications. G) A second run of ablations are completed by repeating steps C-E. H) A final review of the ablated segments after double application.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Benjamin Norton indicated no relevant financial relationships.
Apostolis Papaefthymiou indicated no relevant financial relationships.
Andrea Telese indicated no relevant financial relationships.
Leonardo Rodriguez Grunert: Aqua Medical – Grant/Research Support.
Pablo Hoebel: Aqua Medical – Grant/Research Support.
Paulina Vignoloa: Aqua Medical – Grant/Research Support.
Charles Murray indicated no relevant financial relationships.
Kenneth Chang: Aqua Medical – Grant/Research Support.
C Roberto Simon-Linares indicated no relevant financial relationships.
Rehan Haidry: Aqua Medical – Grant/Research Support. Boston Scientific – Grant/Research Support. Cook medical – Grant/Research Support. Endogastric solutions – Grant/Research Support. Medtronic – Grant/Research Support. Odin vision – Grant/Research Support. Pentax – Grant/Research Support.
Benjamin Norton, MRCP1, Apostolis Papaefthymiou, MD1, Andrea Telese, MD1, Leonardo Rodriguez Grunert, 2, Pablo Hoebel, MD2, Paulina Vignoloa, MD2, Charles Murray, MD1, Kenneth Chang, MD3, C Roberto Simon-Linares, MD4, Rehan Haidry, MD1. P1054 - Radiofrequency Vapor Ablation is Safe and Well-Tolerated for Duodenal Mucosal Ablation in the Treatment of Type 2 Diabetes: Six Month Results From the First-in-Human Dose Finding Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


