Sunday Poster Session
Category: Interventional Endoscopy
P1055 - Dexmedetomidine as an Adjunctive Sedative in Patients Undergoing Endoscopic Submucosal Dissection: A Systematic Review and Meta-analysis.
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio
- AS
Arshia Sethi, MD
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Hazem Abosheaishaa, MD1, Abdallfatah Abdallfatah, 2, Arshia Sethi, MD3, Moataz Aboeldahb, MD4, Abdelmalek Abdelghany, MBBCh5, Abdellatif Ismail, MD6, Islam Mohamed, MD7, Ahmed E. Salem, MBBCh8, Mahmoud Nassar, MD, PhD, MSc, MPA9, Mohammed Abusuliman, MD10, Omar Abdelhalim, MD1, Mohammad Bilal, MD11
1Icahn School of Medicine at Mount Sinai, Queens, NY; 2October 6 University, Cairo, Al Jizah, Egypt; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4Mayo Foundation for Medical Education and Research, Rochester, MN; 5October 6 University, Giza, Al Jizah, Egypt; 6University of Maryland Medical Center, Baltimore, MD; 7University of Missouri, Kansas City, MO; 8Maimonides Medical Center, Brooklyn, NY; 9Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY; 10Henry Ford Health, Detroit, MI; 11University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Endoscopic submucosal dissection (ESD) allows for curative en-bloc resection of dysplastic gastrointestinal (GI) tract lesions. However, it is associated with postoperative adverse events (AEs) such as pain, bleeding, and perforation. Dexmedetomidine, an α2-receptor agonist, has emerged as a promising adjunct sedative for ESD under moderate sedation, offering anxiolysis and analgesia. We conducted a systematic review and meta-analysis to evaluate its efficacy and safety for use in ESD.
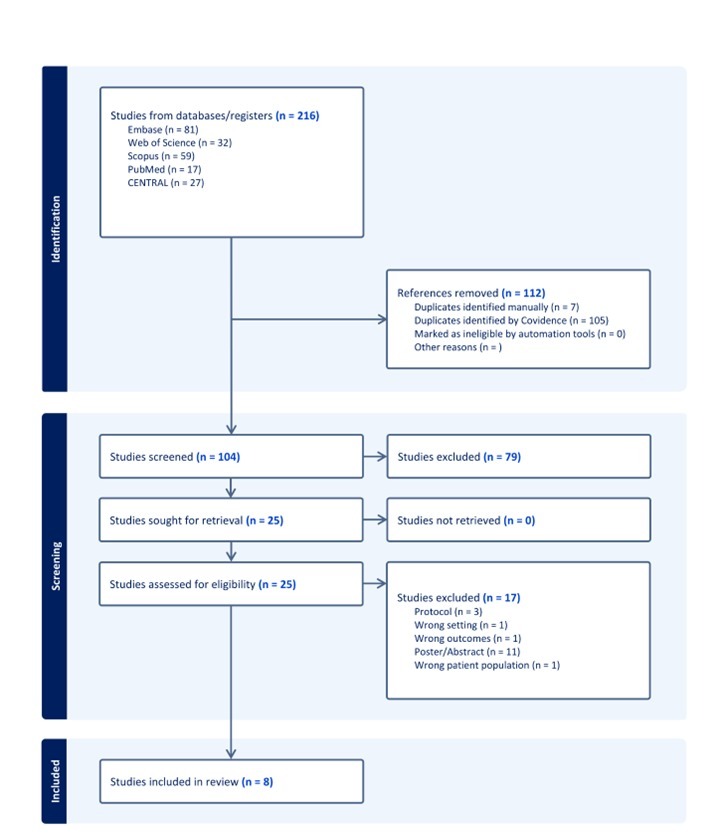
Methods: A comprehensive systematic search was conducted across multiple databases, including Embase, Medline, Scopus, and Web of Science. Studies that involved ESD utilizing dexmedetomidine as an adjunctive medication in combination with other sedatives, were included. Data extraction and risk of bias assessment were independently performed by two reviewers. Meta-analysis was carried out with RevMan using a random-effects model.
Results: Eight studies were included in the final analysis. Dexmedetomidine showed no significant difference in en-bloc or complete resection rates compared to controls. Sedation and procedure times were similar between the two groups as well. Dexmedetomidine significantly reduced restlessness (OR 0.15, 95% CI:0.07 to 0.29) and increased bradycardia (OR 7.15, 95% CI 3.17 to 16.11) compared to controls. Upon subgroup analysis, Dexmedetomidine plus Propofol, and Dexmedetomidine plus Midazolam, revealed the same findings regarding restlessness and bradycardia compared to controls which confirmed the adjunctive effects of Dexmedetomidine.
Discussion: Dexmedetomidine as an adjunctive sedative appears safe and effective in ESD, reducing restlessness without significant adverse events. The risk of bradycardia is increased, which may be reflective of reduced physiological stress. Future studies should explore optimal dosing and compare Dexmedetomidine with other sedatives in diverse populations.
Disclosures:
Hazem Abosheaishaa, MD1, Abdallfatah Abdallfatah, 2, Arshia Sethi, MD3, Moataz Aboeldahb, MD4, Abdelmalek Abdelghany, MBBCh5, Abdellatif Ismail, MD6, Islam Mohamed, MD7, Ahmed E. Salem, MBBCh8, Mahmoud Nassar, MD, PhD, MSc, MPA9, Mohammed Abusuliman, MD10, Omar Abdelhalim, MD1, Mohammad Bilal, MD11. P1055 - Dexmedetomidine as an Adjunctive Sedative in Patients Undergoing Endoscopic Submucosal Dissection: A Systematic Review and Meta-analysis., ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, Queens, NY; 2October 6 University, Cairo, Al Jizah, Egypt; 3Icahn School of Medicine at Mount Sinai, New York, NY; 4Mayo Foundation for Medical Education and Research, Rochester, MN; 5October 6 University, Giza, Al Jizah, Egypt; 6University of Maryland Medical Center, Baltimore, MD; 7University of Missouri, Kansas City, MO; 8Maimonides Medical Center, Brooklyn, NY; 9Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY; 10Henry Ford Health, Detroit, MI; 11University of Minnesota and Minneapolis VA Health Care System, Minneapolis, MN
Introduction: Endoscopic submucosal dissection (ESD) allows for curative en-bloc resection of dysplastic gastrointestinal (GI) tract lesions. However, it is associated with postoperative adverse events (AEs) such as pain, bleeding, and perforation. Dexmedetomidine, an α2-receptor agonist, has emerged as a promising adjunct sedative for ESD under moderate sedation, offering anxiolysis and analgesia. We conducted a systematic review and meta-analysis to evaluate its efficacy and safety for use in ESD.
Methods: A comprehensive systematic search was conducted across multiple databases, including Embase, Medline, Scopus, and Web of Science. Studies that involved ESD utilizing dexmedetomidine as an adjunctive medication in combination with other sedatives, were included. Data extraction and risk of bias assessment were independently performed by two reviewers. Meta-analysis was carried out with RevMan using a random-effects model.
Results: Eight studies were included in the final analysis. Dexmedetomidine showed no significant difference in en-bloc or complete resection rates compared to controls. Sedation and procedure times were similar between the two groups as well. Dexmedetomidine significantly reduced restlessness (OR 0.15, 95% CI:0.07 to 0.29) and increased bradycardia (OR 7.15, 95% CI 3.17 to 16.11) compared to controls. Upon subgroup analysis, Dexmedetomidine plus Propofol, and Dexmedetomidine plus Midazolam, revealed the same findings regarding restlessness and bradycardia compared to controls which confirmed the adjunctive effects of Dexmedetomidine.
Discussion: Dexmedetomidine as an adjunctive sedative appears safe and effective in ESD, reducing restlessness without significant adverse events. The risk of bradycardia is increased, which may be reflective of reduced physiological stress. Future studies should explore optimal dosing and compare Dexmedetomidine with other sedatives in diverse populations.

Figure: PRISMA flow chart for search strategy
Disclosures:
Hazem Abosheaishaa indicated no relevant financial relationships.
Abdallfatah Abdallfatah indicated no relevant financial relationships.
Arshia Sethi indicated no relevant financial relationships.
Moataz Aboeldahb indicated no relevant financial relationships.
Abdelmalek Abdelghany indicated no relevant financial relationships.
Abdellatif Ismail indicated no relevant financial relationships.
Islam Mohamed indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Mahmoud Nassar indicated no relevant financial relationships.
Mohammed Abusuliman indicated no relevant financial relationships.
Omar Abdelhalim indicated no relevant financial relationships.
Mohammad Bilal: Boston Scientific – Consultant. Cook endoscopy – Speakers Bureau.
Hazem Abosheaishaa, MD1, Abdallfatah Abdallfatah, 2, Arshia Sethi, MD3, Moataz Aboeldahb, MD4, Abdelmalek Abdelghany, MBBCh5, Abdellatif Ismail, MD6, Islam Mohamed, MD7, Ahmed E. Salem, MBBCh8, Mahmoud Nassar, MD, PhD, MSc, MPA9, Mohammed Abusuliman, MD10, Omar Abdelhalim, MD1, Mohammad Bilal, MD11. P1055 - Dexmedetomidine as an Adjunctive Sedative in Patients Undergoing Endoscopic Submucosal Dissection: A Systematic Review and Meta-analysis., ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
