Sunday Poster Session
Category: Liver
P1133 - Tirzepatide vs Semaglutide in Obese Patients With MAFLD or MASH: A Comparative Analysis of Cardiovascular and Gastrointestinal Outcomes, and the Risk of Progression to Fibrosis or Hepatocellular Carcinoma
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Olanrewaju Adeniran, MBBS
West Virginia University School of Medicine
Morgantown, WV
Presenting Author(s)
Olanrewaju Adeniran, MBBS1, Luis M.. Nieto, MD2, Ifeoma P. Kwentoh, MD3, Joshua Kirkpatrick, MD1, Ayowumi Adekolu, MD1, Ethan M.. Cohen, MD4, Kanith Farah, MD1, Farirai Marwizi, MD5, Sharon Narvaez, MD6, Do Han Kim, MD7, Donghyun Ko, MD8, Islam Younes, MD1
1West Virginia University School of Medicine, Morgantown, WV; 2Emory University School of Medicine, Atlanta, GA; 3Harlem Hospital Center, New York, NY; 4West Virginia University, Morgantown, WV; 5St. John's Medical College and Hospital, Rockaway Park, NY; 6Universidad de Guayaquil, School of Medicine, Atlanta, GA; 7Mount Sinai Morningside and West, Icahn School of Medicine at Mount Sinai, New York, NY; 8Yale-New Haven Health/Bridgeport Hospital, Bridgeport, CT
Introduction: Non-alcoholic steatotic liver disease (SLD), which comprises metabolic dysfunction-associated fatty liver disease (MAFLD) and metabolic dysfunction-associated steatohepatitis (MASH), have been associated with increased cardiovascular and cerebrovascular risks and progression to hepatic fibrosis or hepatocellular carcinoma (HCC). Semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), and Tirzepatide, a combined GLP-1 RA and Glucose-dependent insulinotropic polypeptide receptor agonist (GIP RA), have shown promising results in treating MAFLD and MASH, despite their gastrointestinal (GI) side effects. Limited studies have compared the cardiovascular and cerebrovascular outcomes, the GI effects, and the risk of progression to fibrosis or HCC development in obese patients with MAFLD or MASH on either medication. We aim to evaluate these events.
Methods: This large, retrospective, population-based cohort study utilizes data from the TriNetX platform. Adult 18 years and above, BMI 30 or higher with MAFLD or MASH treated with Tirzepatide or Semaglutide between January 1, 2020, and May 30, 2024, were included. Patients who were pregnant, on other GLP-1 RA, diagnosed with SLD before and up to 3 months after initiation of either medication were excluded. The cohorts were matched according to demographics, related liver diseases, comorbidities, medications, and laboratory results using 1:1 propensity score matching (PSM).
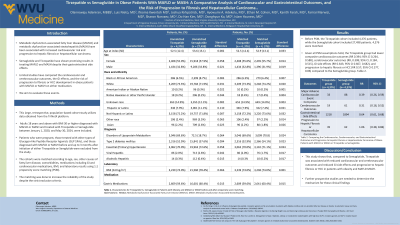
Results: Before PSM, the Tirzepatide cohort included 4,376 patients, while the Semaglutide cohort included 27,498 patients. 4 376 patients were matched. Given that all PSM assumptions hold, the Tirzepatide group had significantly lower composite cardiovascular outcomes (RR 0.394; 95% CI 0.264, 0.588), composite cerebrovascular outcomes (RR, 0.308; 95% CI, 0.184, 0.515), GI side effects (RR 0.643; 95% CI 0.607, 0.682), and progression to hepatic fibrosis or HCC (RR 0.57; 95% CI 0.377, 0.86) compared to the Semaglutide group. (Table 1)
Discussion: This study shows that when compared to Semaglutide, Tirzepatide was associated with reduced cardiovascular and cerebrovascular outcomes, GI side effects, and reduced progression to hepatic fibrosis or HCC in adult patients with obesity and MAFLD or MASH. Further prospective studies are needed to determine the mechanism for these clinical findings
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Olanrewaju Adeniran, MBBS1, Luis M.. Nieto, MD2, Ifeoma P. Kwentoh, MD3, Joshua Kirkpatrick, MD1, Ayowumi Adekolu, MD1, Ethan M.. Cohen, MD4, Kanith Farah, MD1, Farirai Marwizi, MD5, Sharon Narvaez, MD6, Do Han Kim, MD7, Donghyun Ko, MD8, Islam Younes, MD1. P1133 - Tirzepatide vs Semaglutide in Obese Patients With MAFLD or MASH: A Comparative Analysis of Cardiovascular and Gastrointestinal Outcomes, and the Risk of Progression to Fibrosis or Hepatocellular Carcinoma, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1West Virginia University School of Medicine, Morgantown, WV; 2Emory University School of Medicine, Atlanta, GA; 3Harlem Hospital Center, New York, NY; 4West Virginia University, Morgantown, WV; 5St. John's Medical College and Hospital, Rockaway Park, NY; 6Universidad de Guayaquil, School of Medicine, Atlanta, GA; 7Mount Sinai Morningside and West, Icahn School of Medicine at Mount Sinai, New York, NY; 8Yale-New Haven Health/Bridgeport Hospital, Bridgeport, CT
Introduction: Non-alcoholic steatotic liver disease (SLD), which comprises metabolic dysfunction-associated fatty liver disease (MAFLD) and metabolic dysfunction-associated steatohepatitis (MASH), have been associated with increased cardiovascular and cerebrovascular risks and progression to hepatic fibrosis or hepatocellular carcinoma (HCC). Semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), and Tirzepatide, a combined GLP-1 RA and Glucose-dependent insulinotropic polypeptide receptor agonist (GIP RA), have shown promising results in treating MAFLD and MASH, despite their gastrointestinal (GI) side effects. Limited studies have compared the cardiovascular and cerebrovascular outcomes, the GI effects, and the risk of progression to fibrosis or HCC development in obese patients with MAFLD or MASH on either medication. We aim to evaluate these events.
Methods: This large, retrospective, population-based cohort study utilizes data from the TriNetX platform. Adult 18 years and above, BMI 30 or higher with MAFLD or MASH treated with Tirzepatide or Semaglutide between January 1, 2020, and May 30, 2024, were included. Patients who were pregnant, on other GLP-1 RA, diagnosed with SLD before and up to 3 months after initiation of either medication were excluded. The cohorts were matched according to demographics, related liver diseases, comorbidities, medications, and laboratory results using 1:1 propensity score matching (PSM).
Results: Before PSM, the Tirzepatide cohort included 4,376 patients, while the Semaglutide cohort included 27,498 patients. 4 376 patients were matched. Given that all PSM assumptions hold, the Tirzepatide group had significantly lower composite cardiovascular outcomes (RR 0.394; 95% CI 0.264, 0.588), composite cerebrovascular outcomes (RR, 0.308; 95% CI, 0.184, 0.515), GI side effects (RR 0.643; 95% CI 0.607, 0.682), and progression to hepatic fibrosis or HCC (RR 0.57; 95% CI 0.377, 0.86) compared to the Semaglutide group. (Table 1)
Discussion: This study shows that when compared to Semaglutide, Tirzepatide was associated with reduced cardiovascular and cerebrovascular outcomes, GI side effects, and reduced progression to hepatic fibrosis or HCC in adult patients with obesity and MAFLD or MASH. Further prospective studies are needed to determine the mechanism for these clinical findings
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Olanrewaju Adeniran indicated no relevant financial relationships.
Luis Nieto indicated no relevant financial relationships.
Ifeoma Kwentoh indicated no relevant financial relationships.
Joshua Kirkpatrick indicated no relevant financial relationships.
Ayowumi Adekolu indicated no relevant financial relationships.
Ethan Cohen indicated no relevant financial relationships.
Kanith Farah indicated no relevant financial relationships.
Farirai Marwizi indicated no relevant financial relationships.
Sharon Narvaez indicated no relevant financial relationships.
Do Han Kim indicated no relevant financial relationships.
Donghyun Ko indicated no relevant financial relationships.
Islam Younes indicated no relevant financial relationships.
Olanrewaju Adeniran, MBBS1, Luis M.. Nieto, MD2, Ifeoma P. Kwentoh, MD3, Joshua Kirkpatrick, MD1, Ayowumi Adekolu, MD1, Ethan M.. Cohen, MD4, Kanith Farah, MD1, Farirai Marwizi, MD5, Sharon Narvaez, MD6, Do Han Kim, MD7, Donghyun Ko, MD8, Islam Younes, MD1. P1133 - Tirzepatide vs Semaglutide in Obese Patients With MAFLD or MASH: A Comparative Analysis of Cardiovascular and Gastrointestinal Outcomes, and the Risk of Progression to Fibrosis or Hepatocellular Carcinoma, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
