Sunday Poster Session
Category: Pediatrics
P1472 - Prescription Patterns of Glucagon-Like Peptide-1 Receptor Agonists in the Pediatric Population
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Danae Black, PhD, MPH
OMNY Health
Atlanta, GA
Presenting Author(s)
Danae Black, PhD, MPH, Lawrence Rasouliyan, MPH, Stacey Long, MS
OMNY Health, Atlanta, GA
Introduction: Pediatric use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) is increasing in the United States; however, research is limited due to safety and regulatory barriers. Also, patients must overcome barriers to access due to diagnostic requirements and high costs. Understanding current treatment approaches among patients under the age of 18 is important to inform future treatment recommendations as GLP-1 RA use continues to increase.
Methods: Electronic health record data from 2017 – 2024 from the OMNY Health real-world data platform were accessed, and individuals under the age of 18 who received semaglutide (for weight loss or treatment of type 2 diabetes [T2D]) or tirzepatide were selected. Diagnoses, encounters, prescription orders and administration, and vitals among selected patients were included. Demographic characteristics were evaluated at the earliest age of index prescription. Treatments were evaluated after the index prescription.
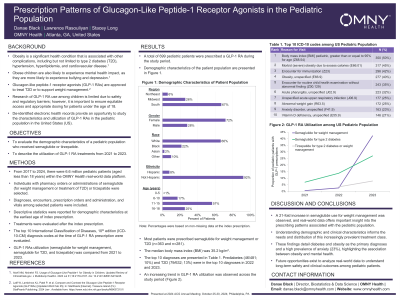
Results: A total of 699 pediatric patients were prescribed GLP-1 RA during the study period. The median age was 16 years and most patients were female (72%). The racial distribution of the population was as follows: white (70%), black or African American (23%), and other racial categories (5%). Most of the study population was geographically concentrated in the South (67%).
Most patients took semaglutide for weight loss or T2D (n=363 and n=281). The median body mass index (BMI) was 35.2, and the top diagnoses among pediatric patients on GLP-1 RAs were obesity-related (BMI > 95th percentile, morbid (severe) obesity due to excess calories, and obesity, unspecified). Prediabetes (46/481; 10%) and T2D (79/632; 13%) were in the top 10 diagnoses in 2022 and 2023. An increasing trend in GLP-1 RA utilization was observed, with the largest increase occurring from 2022 to 2023. Specifically, a 1.6-, 2.0-, and 21.1-fold increase in semaglutide for T2D, tirzepatide, and semaglutide for weight loss, respectively, from 2022 to 2023.
Discussion: This study provides important insight into the demographic and clinical characteristics of pediatric patients prescribed GLP-1 RAs in the United States. These findings highlight diabetes and obesity as the primary diagnoses associated with GLP-1 use among pediatric patients in our cohort and the growing prevalence of both diseases and treatment utilization. Additional research to understand long-term safety and clinical outcomes is important to continue understanding the management of obesity among pediatric patients.
Disclosures:
Danae Black, PhD, MPH, Lawrence Rasouliyan, MPH, Stacey Long, MS. P1472 - Prescription Patterns of Glucagon-Like Peptide-1 Receptor Agonists in the Pediatric Population, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
OMNY Health, Atlanta, GA
Introduction: Pediatric use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) is increasing in the United States; however, research is limited due to safety and regulatory barriers. Also, patients must overcome barriers to access due to diagnostic requirements and high costs. Understanding current treatment approaches among patients under the age of 18 is important to inform future treatment recommendations as GLP-1 RA use continues to increase.
Methods: Electronic health record data from 2017 – 2024 from the OMNY Health real-world data platform were accessed, and individuals under the age of 18 who received semaglutide (for weight loss or treatment of type 2 diabetes [T2D]) or tirzepatide were selected. Diagnoses, encounters, prescription orders and administration, and vitals among selected patients were included. Demographic characteristics were evaluated at the earliest age of index prescription. Treatments were evaluated after the index prescription.
Results: A total of 699 pediatric patients were prescribed GLP-1 RA during the study period. The median age was 16 years and most patients were female (72%). The racial distribution of the population was as follows: white (70%), black or African American (23%), and other racial categories (5%). Most of the study population was geographically concentrated in the South (67%).
Most patients took semaglutide for weight loss or T2D (n=363 and n=281). The median body mass index (BMI) was 35.2, and the top diagnoses among pediatric patients on GLP-1 RAs were obesity-related (BMI > 95th percentile, morbid (severe) obesity due to excess calories, and obesity, unspecified). Prediabetes (46/481; 10%) and T2D (79/632; 13%) were in the top 10 diagnoses in 2022 and 2023. An increasing trend in GLP-1 RA utilization was observed, with the largest increase occurring from 2022 to 2023. Specifically, a 1.6-, 2.0-, and 21.1-fold increase in semaglutide for T2D, tirzepatide, and semaglutide for weight loss, respectively, from 2022 to 2023.
Discussion: This study provides important insight into the demographic and clinical characteristics of pediatric patients prescribed GLP-1 RAs in the United States. These findings highlight diabetes and obesity as the primary diagnoses associated with GLP-1 use among pediatric patients in our cohort and the growing prevalence of both diseases and treatment utilization. Additional research to understand long-term safety and clinical outcomes is important to continue understanding the management of obesity among pediatric patients.

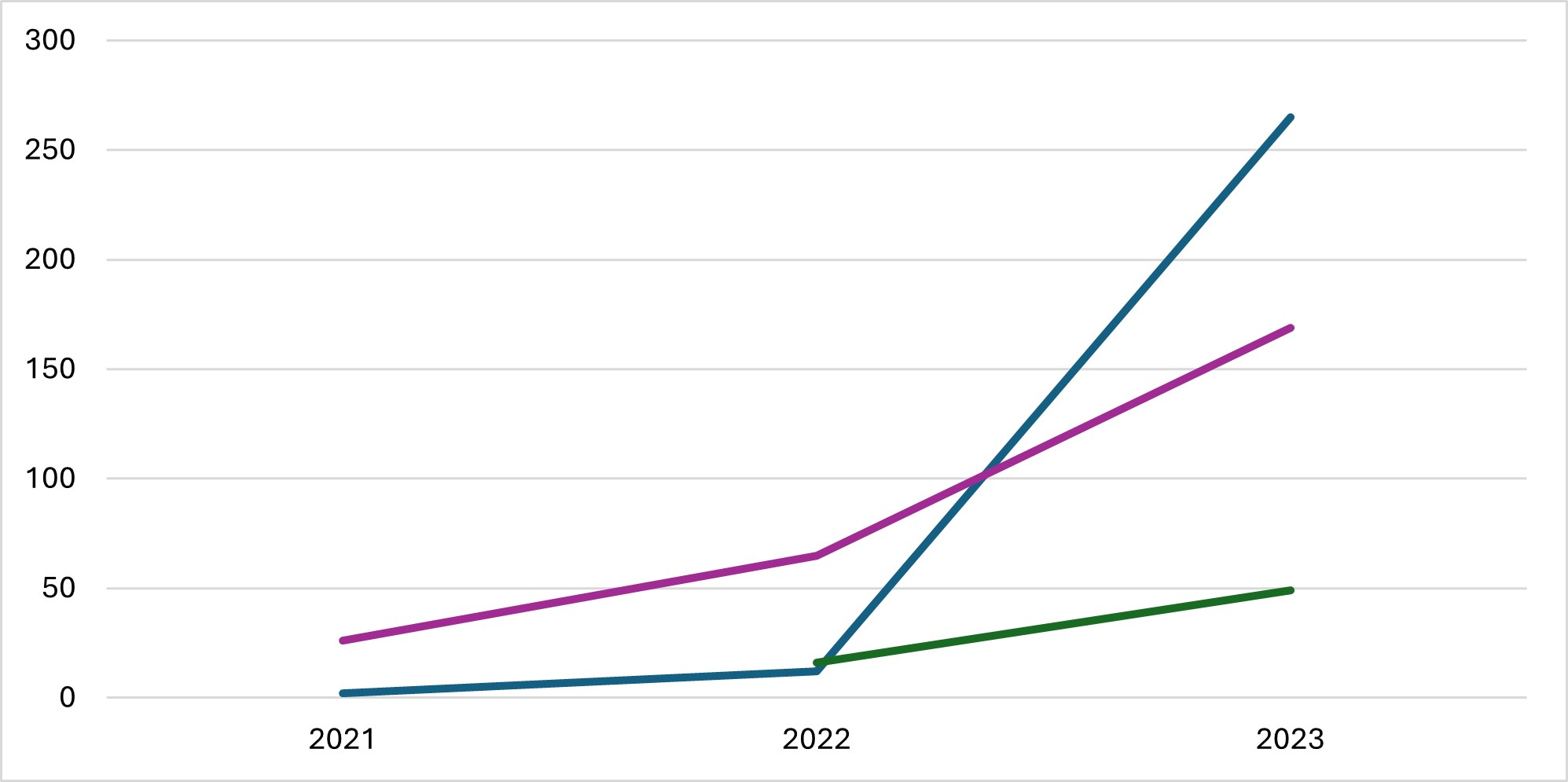
Figure: Pediatric Patients Using Glucagon-like Peptide-1 Receptor Agonists, 2021-2023. Blue = semaglutide for weight loss; Green = tirzepatide ; Purple = semaglutide for type 2 diabetes. There is an upward trend across all treatment types, with the largest change in GLP-1 RA use among pediatric patients taking semaglutide for weight loss.
Disclosures:
Danae Black indicated no relevant financial relationships.
Lawrence Rasouliyan indicated no relevant financial relationships.
Stacey Long indicated no relevant financial relationships.
Danae Black, PhD, MPH, Lawrence Rasouliyan, MPH, Stacey Long, MS. P1472 - Prescription Patterns of Glucagon-Like Peptide-1 Receptor Agonists in the Pediatric Population, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
