Sunday Poster Session
Category: Stomach
P1667 - Pembrolizumab-Induced Gastritis: A Call for Recognition
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Jameel Alp, MD
University of Minnesota
Minnetonka, MN
Presenting Author(s)
Jameel Alp, MD1, Rahul Karna, MD2, Khalid Amin, MD2, Joshua A. Sloan, DO2
1University of Minnesota, Minnetonka, MN; 2University of Minnesota, Minneapolis, MN
Introduction: While pembrolizumab has significantly advanced cancer treatment, its use can lead to immune-related adverse events (irAEs). Although colitis, hepatitis, and pancreatitis are well-known irAEs, gastritis should also be recognized as a potential irAE.
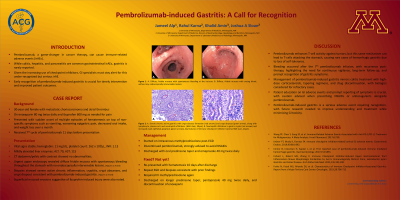
Case Description/Methods: A 30-year-old female with metastatic choriocarcinoma and atrial thrombus on enoxaparin presented with sudden-onset hematemesis. She had been using ibuprofen for pain relief. 11 days prior, she received her 7th cycle of pembrolizumab. On admission, vitals were stable. Abnormal labs included hemoglobin of 11 mg/dL, INR of 1.13, ALT of 70 U/L, and AST of 115 U/L. Imaging was unremarkable. Urgent esophagogastroduodenoscopy (EGD) revealed diffuse friable mucosa with spontaneous bleeding throughout the stomach. Biopsies showed severe active chronic inflammation consistent with pembrolizumab-induced gastritis and superficial mucosal erosions suggestive of nonsteroidal anti-inflammatory drug (NSAID)-induced injury. Pembrolizumab was discontinued, and the patient received intravenous (IV) methylprednisolone, followed by a prednisone taper and omeprazole. She was readmitted 10 days later with recurrent hematemesis, requiring repeat EGD and IV methylprednisolone. She was discharged on a longer prednisone taper, pantoprazole, and discontinuation of enoxaparin.
Discussion: Pembrolizumab heightens T-cell response against tumors but may trigger immune-related gastritis, leading to severe hemorrhage. What is even more concerning is that the onset of pembrolizumab-induced gastritis (PG) is unpredictable, requiring continuous vigilance and careful symptom monitoring. Its potential for recurrence also necessitates long-term follow-up. Patient education on recognizing symptoms and avoiding exacerbating factors like NSAIDs is crucial. Given the wide use of checkpoint inhibitors, it will be important to keep PG in the differential in patients undergoing treatment with the drug presenting with symptoms that could be related to therapy.
Disclosures:
Jameel Alp, MD1, Rahul Karna, MD2, Khalid Amin, MD2, Joshua A. Sloan, DO2. P1667 - Pembrolizumab-Induced Gastritis: A Call for Recognition, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Minnesota, Minnetonka, MN; 2University of Minnesota, Minneapolis, MN
Introduction: While pembrolizumab has significantly advanced cancer treatment, its use can lead to immune-related adverse events (irAEs). Although colitis, hepatitis, and pancreatitis are well-known irAEs, gastritis should also be recognized as a potential irAE.
Case Description/Methods: A 30-year-old female with metastatic choriocarcinoma and atrial thrombus on enoxaparin presented with sudden-onset hematemesis. She had been using ibuprofen for pain relief. 11 days prior, she received her 7th cycle of pembrolizumab. On admission, vitals were stable. Abnormal labs included hemoglobin of 11 mg/dL, INR of 1.13, ALT of 70 U/L, and AST of 115 U/L. Imaging was unremarkable. Urgent esophagogastroduodenoscopy (EGD) revealed diffuse friable mucosa with spontaneous bleeding throughout the stomach. Biopsies showed severe active chronic inflammation consistent with pembrolizumab-induced gastritis and superficial mucosal erosions suggestive of nonsteroidal anti-inflammatory drug (NSAID)-induced injury. Pembrolizumab was discontinued, and the patient received intravenous (IV) methylprednisolone, followed by a prednisone taper and omeprazole. She was readmitted 10 days later with recurrent hematemesis, requiring repeat EGD and IV methylprednisolone. She was discharged on a longer prednisone taper, pantoprazole, and discontinuation of enoxaparin.
Discussion: Pembrolizumab heightens T-cell response against tumors but may trigger immune-related gastritis, leading to severe hemorrhage. What is even more concerning is that the onset of pembrolizumab-induced gastritis (PG) is unpredictable, requiring continuous vigilance and careful symptom monitoring. Its potential for recurrence also necessitates long-term follow-up. Patient education on recognizing symptoms and avoiding exacerbating factors like NSAIDs is crucial. Given the wide use of checkpoint inhibitors, it will be important to keep PG in the differential in patients undergoing treatment with the drug presenting with symptoms that could be related to therapy.
Disclosures:
Jameel Alp indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Khalid Amin indicated no relevant financial relationships.
Joshua Sloan: Sanofi-Regeneron – Advisory Committee/Board Member, Speakers Bureau.
Jameel Alp, MD1, Rahul Karna, MD2, Khalid Amin, MD2, Joshua A. Sloan, DO2. P1667 - Pembrolizumab-Induced Gastritis: A Call for Recognition, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
