Sunday Poster Session
Category: Stomach
P1606 - Safety of GLP-1 Agonists With Metoclopramide Nasal Spray in Women With Diabetic Gastroparesis: Insights From a Phase 3 Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Richard W. McCallum, MD
Texas Tech University Health Sciences Center School of Medicine
El Paso, TX
Presenting Author(s)
Richard W. McCallum, MD1, Marilyn R. Carlson, MD2, David C. Kunkel, MD3
1Texas Tech University Health Sciences Center School of Medicine, El Paso, TX; 2Evoke Pharma, Solana Beach, CA; 3University of California San Diego, La Jolla, CA
Introduction: Metoclopramide nasal spray is FDA-approved for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. GLP-1 receptor agonists, commonly used for diabetes and weight loss, may cause side effects such as nausea and vomiting due to their mechanism of action, which delays gastric emptying. Given the elimination half-life of these drugs, symptoms suggestive of gastroparesis may persist for weeks after discontinuation. This study aims to evaluate the safety of using metoclopramide nasal spray (MNS) concomitantly with GLP-1 drugs in women with diabetic gastroparesis and confirmed delayed gastric emptying.
Methods: This post-hoc analysis reviewed data from a 2016 Phase 3 study (N=205). The subset analyzed included 36 patients using GLP-1 drugs during the 28-day study; 23 on placebo and 13 on MNS 10 mg. The average age was 51.4 years (range 32-72), with 67% identified as Caucasian and 33% as Black. The GLP-1 drugs were those available in 2016 and included; liraglutide, canagliflozin, exenatide, dapagliflozin, empagliflozin, and repaglinide. Three patients on MNS used two GLP-1 drugs.
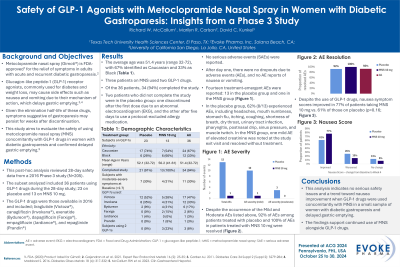
Results: Of the 36 patients, 34 (94%) completed the study. Two patients who did not complete the study were in the placebo group: one discontinued after the first dose due to an abnormal EKG, and the other after five days to use a protocol-excluded allergy medication. No serious adverse events (SAEs) were reported. After day one, there were no dropouts due to adverse events (AEs), and no AE reports of nausea or vomiting. Fourteen treatment-emergent AEs were reported: 13 in the placebo group and one in the MNS group. In the placebo group, 62% (8/13) experienced AEs, including headaches, mouth numbness, stomach flu, itching, coughing, shortness of breath, dry throat, urinary tract infection, pharyngitis, postnasal drip, sinus pressure, and muscle twitch. In the MNS group, one mild AE of elevated creatinine was noted at the study exit visit and resolved without treatment. Despite the use of GLP-1 drugs, nausea symptom scores improved in 77% of patients taking MNS vs. 61% of those on placebo (p=0.16).
Discussion: This analysis indicates no serious safety issues and a trend toward nausea improvement when GLP 1 drugs were used concomitantly with MNS in a small sample of women with diabetic gastroparesis and delayed gastric emptying. The findings support continued use of MNS alongside GLP-1 drugs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Richard W. McCallum, MD1, Marilyn R. Carlson, MD2, David C. Kunkel, MD3. P1606 - Safety of GLP-1 Agonists With Metoclopramide Nasal Spray in Women With Diabetic Gastroparesis: Insights From a Phase 3 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Texas Tech University Health Sciences Center School of Medicine, El Paso, TX; 2Evoke Pharma, Solana Beach, CA; 3University of California San Diego, La Jolla, CA
Introduction: Metoclopramide nasal spray is FDA-approved for the relief of symptoms in adults with acute and recurrent diabetic gastroparesis. GLP-1 receptor agonists, commonly used for diabetes and weight loss, may cause side effects such as nausea and vomiting due to their mechanism of action, which delays gastric emptying. Given the elimination half-life of these drugs, symptoms suggestive of gastroparesis may persist for weeks after discontinuation. This study aims to evaluate the safety of using metoclopramide nasal spray (MNS) concomitantly with GLP-1 drugs in women with diabetic gastroparesis and confirmed delayed gastric emptying.
Methods: This post-hoc analysis reviewed data from a 2016 Phase 3 study (N=205). The subset analyzed included 36 patients using GLP-1 drugs during the 28-day study; 23 on placebo and 13 on MNS 10 mg. The average age was 51.4 years (range 32-72), with 67% identified as Caucasian and 33% as Black. The GLP-1 drugs were those available in 2016 and included; liraglutide, canagliflozin, exenatide, dapagliflozin, empagliflozin, and repaglinide. Three patients on MNS used two GLP-1 drugs.
Results: Of the 36 patients, 34 (94%) completed the study. Two patients who did not complete the study were in the placebo group: one discontinued after the first dose due to an abnormal EKG, and the other after five days to use a protocol-excluded allergy medication. No serious adverse events (SAEs) were reported. After day one, there were no dropouts due to adverse events (AEs), and no AE reports of nausea or vomiting. Fourteen treatment-emergent AEs were reported: 13 in the placebo group and one in the MNS group. In the placebo group, 62% (8/13) experienced AEs, including headaches, mouth numbness, stomach flu, itching, coughing, shortness of breath, dry throat, urinary tract infection, pharyngitis, postnasal drip, sinus pressure, and muscle twitch. In the MNS group, one mild AE of elevated creatinine was noted at the study exit visit and resolved without treatment. Despite the use of GLP-1 drugs, nausea symptom scores improved in 77% of patients taking MNS vs. 61% of those on placebo (p=0.16).
Discussion: This analysis indicates no serious safety issues and a trend toward nausea improvement when GLP 1 drugs were used concomitantly with MNS in a small sample of women with diabetic gastroparesis and delayed gastric emptying. The findings support continued use of MNS alongside GLP-1 drugs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Richard McCallum: Evoke Pharma – Consultant.
Marilyn Carlson: Evoke Pharma – Employee. Evoke Pharma – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds).
David Kunkel: Evoke Pharma – Consultant. Phathom Pharmaceuticals – Speakers Bureau. Regeneron Pharmaceuticals – Speakers Bureau. Sanofi – Speakers Bureau. Vanda Pharmaceuticals – Grant/Research Support.
Richard W. McCallum, MD1, Marilyn R. Carlson, MD2, David C. Kunkel, MD3. P1606 - Safety of GLP-1 Agonists With Metoclopramide Nasal Spray in Women With Diabetic Gastroparesis: Insights From a Phase 3 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
