Sunday Poster Session
Category: Small Intestine
P1521 - Safety and Tolerability of Once-Weekly Glucagon-Like Peptide-2 Analog Apraglutide in Patients With Short Bowel Syndrome and Intestinal Failure: Results From a Global, Phase 3, Randomized, Double-Blind Trial
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio
- MB
Mena Boules, MD
Ironwood Pharmaceuticals, Inc.
Boston, MA
Presenting Author(s)
Award: Presidential Poster Award
Tim Vanuytsel, MD, PhD1, Simon Lal, MD, PhD2, Kelly Tappenden, PhD, RD3, Donald Kirby, MD4, Palle Jeppesen, MD, PhD5, Francisca Joly, MD, PhD6, Tomasz Masior, MD7, Patricia Valencia, PharmD7, Chang Ming, MS, PhD7, Mena Boules, MD8, Susanna Huh, MD, MPH7, Kishore Iyer, MD, MBBS9
1Leuven Intestinal Failure and Transplantation Center, University Hospital, Leuven, Brussels Hoofdstedelijk Gewest, Belgium; 2University of Manchester Manchester, Manchester, England, United Kingdom; 3The University of Utah, Salt Lake City, UT; 4Cleveland Clinic, Cleveland, OH; 5Rigshospitalet, Copenhagen University Hospital, Copenhagen, Sjelland, Denmark; 6Centre for Intestinal Failure, Hôpital Beaujon, Paris, Ile-de-France, France; 7Ironwood Pharmaceuticals, Boston, MA; 8Ironwood Pharmaceuticals, Inc., Boston, MA; 9Mount Sinai Medical Center, New York, NY
Introduction: Apraglutide (APRA), a once-weekly, long-acting glucagon-like peptide-2 (GLP-2) analog, stimulates intestinal adaptation and shows potential to reduce parenteral support (PS) requirements in pts with SBS-IF. The global, Phase 3, double-blind, placebo (PBO)-controlled trial, STARS, met its primary efficacy endpoint of relative change from baseline in weekly PS volume at Week (Wk) 24 in the overall population. The objective of this analysis is to assess the safety and tolerability of APRA in pts with SBS-IF.
Methods: Safety data from the STARS trial (NCT04627025) were evaluated. 164 pts were randomized 2:1 to receive once-weekly subcutaneous APRA (5 mg if pt ≥50 kg, 2.5 mg if pt < 50kg) or PBO, stratified by intestinal anatomy (stoma vs colon-in-continuity [CIC]). Safety endpoints were investigated through Wk 24 in the overall population and Wk 48 in the CIC stratum. Adverse events (AEs) were collected throughout the trial; safety assessments were performed during the trial and at 4 wks after the last dose. The Safety Analysis Set included all pts exposed to APRA or PBO (N=163).
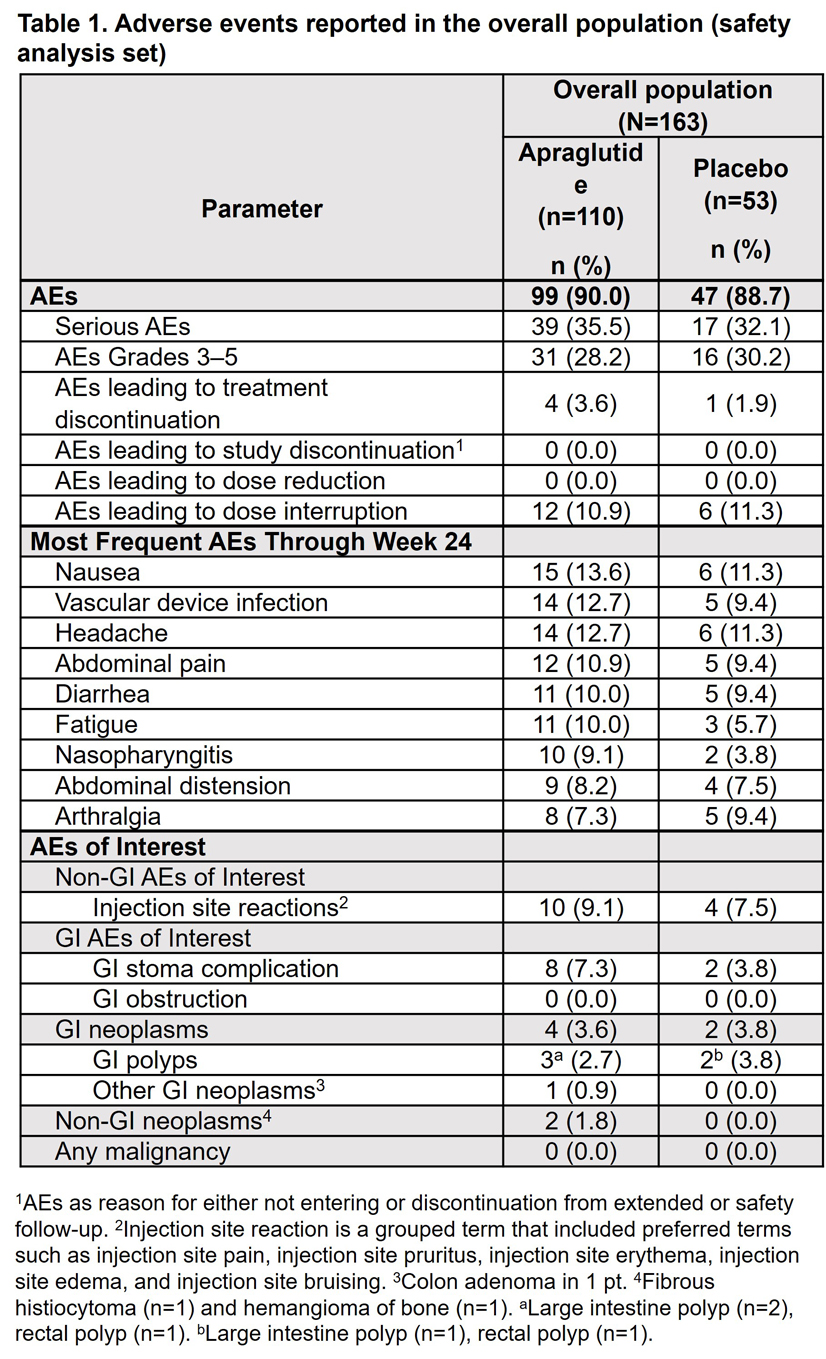
Results: Incidence of AEs, serious AEs, and AEs of Grade ≥3 was similar between treatment arms (Table 1). Dose interruptions due to AEs were also similar between APRA and PBO (10.9% vs 11.3%). The incidence of AEs associated with gastrointestinal (GI) tolerability was low and similar between treatment arms. Most frequently reported AE was nausea (APRA 13.6% vs PBO 11.3%). Incidence of GI polyps was also low (APRA n=3 [2.7%] vs PBO n=2 [3.8%]); all polyps were resolved, with no APRA discontinuations due to GI tolerability symptoms or GI polyps. GI stoma complication was reported in 7.3% of APRA vs 3.8% of PBO pts. There were no cases of GI obstruction or malignancies. Incidence of injection site reactions was similar between APRA and type of event. There was one death in the APRA arm (sudden cardiac death, unrelated). More than 98% of pts had ≥80% treatment compliance in both treatment arms. Most pts entered the long-term, open-label STARS Extend trial at the end of the study (APRA 92% vs PBO 89%).
Discussion: Apraglutide has a safety profile consistent with previous APRA studies, with no new safety concerns. The low incidences of injection site reactions and AEs associated with GI tolerability, along with high treatment compliance, support the safety and tolerability profile of APRA.
Disclosures:
Tim Vanuytsel, MD, PhD1, Simon Lal, MD, PhD2, Kelly Tappenden, PhD, RD3, Donald Kirby, MD4, Palle Jeppesen, MD, PhD5, Francisca Joly, MD, PhD6, Tomasz Masior, MD7, Patricia Valencia, PharmD7, Chang Ming, MS, PhD7, Mena Boules, MD8, Susanna Huh, MD, MPH7, Kishore Iyer, MD, MBBS9. P1521 - Safety and Tolerability of Once-Weekly Glucagon-Like Peptide-2 Analog Apraglutide in Patients With Short Bowel Syndrome and Intestinal Failure: Results From a Global, Phase 3, Randomized, Double-Blind Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Tim Vanuytsel, MD, PhD1, Simon Lal, MD, PhD2, Kelly Tappenden, PhD, RD3, Donald Kirby, MD4, Palle Jeppesen, MD, PhD5, Francisca Joly, MD, PhD6, Tomasz Masior, MD7, Patricia Valencia, PharmD7, Chang Ming, MS, PhD7, Mena Boules, MD8, Susanna Huh, MD, MPH7, Kishore Iyer, MD, MBBS9
1Leuven Intestinal Failure and Transplantation Center, University Hospital, Leuven, Brussels Hoofdstedelijk Gewest, Belgium; 2University of Manchester Manchester, Manchester, England, United Kingdom; 3The University of Utah, Salt Lake City, UT; 4Cleveland Clinic, Cleveland, OH; 5Rigshospitalet, Copenhagen University Hospital, Copenhagen, Sjelland, Denmark; 6Centre for Intestinal Failure, Hôpital Beaujon, Paris, Ile-de-France, France; 7Ironwood Pharmaceuticals, Boston, MA; 8Ironwood Pharmaceuticals, Inc., Boston, MA; 9Mount Sinai Medical Center, New York, NY
Introduction: Apraglutide (APRA), a once-weekly, long-acting glucagon-like peptide-2 (GLP-2) analog, stimulates intestinal adaptation and shows potential to reduce parenteral support (PS) requirements in pts with SBS-IF. The global, Phase 3, double-blind, placebo (PBO)-controlled trial, STARS, met its primary efficacy endpoint of relative change from baseline in weekly PS volume at Week (Wk) 24 in the overall population. The objective of this analysis is to assess the safety and tolerability of APRA in pts with SBS-IF.
Methods: Safety data from the STARS trial (NCT04627025) were evaluated. 164 pts were randomized 2:1 to receive once-weekly subcutaneous APRA (5 mg if pt ≥50 kg, 2.5 mg if pt < 50kg) or PBO, stratified by intestinal anatomy (stoma vs colon-in-continuity [CIC]). Safety endpoints were investigated through Wk 24 in the overall population and Wk 48 in the CIC stratum. Adverse events (AEs) were collected throughout the trial; safety assessments were performed during the trial and at 4 wks after the last dose. The Safety Analysis Set included all pts exposed to APRA or PBO (N=163).
Results: Incidence of AEs, serious AEs, and AEs of Grade ≥3 was similar between treatment arms (Table 1). Dose interruptions due to AEs were also similar between APRA and PBO (10.9% vs 11.3%). The incidence of AEs associated with gastrointestinal (GI) tolerability was low and similar between treatment arms. Most frequently reported AE was nausea (APRA 13.6% vs PBO 11.3%). Incidence of GI polyps was also low (APRA n=3 [2.7%] vs PBO n=2 [3.8%]); all polyps were resolved, with no APRA discontinuations due to GI tolerability symptoms or GI polyps. GI stoma complication was reported in 7.3% of APRA vs 3.8% of PBO pts. There were no cases of GI obstruction or malignancies. Incidence of injection site reactions was similar between APRA and type of event. There was one death in the APRA arm (sudden cardiac death, unrelated). More than 98% of pts had ≥80% treatment compliance in both treatment arms. Most pts entered the long-term, open-label STARS Extend trial at the end of the study (APRA 92% vs PBO 89%).
Discussion: Apraglutide has a safety profile consistent with previous APRA studies, with no new safety concerns. The low incidences of injection site reactions and AEs associated with GI tolerability, along with high treatment compliance, support the safety and tolerability profile of APRA.

Figure: Table 1. Adverse events reported in the overall population (safety analysis set)
Disclosures:
Tim Vanuytsel: Abbott Nutrition Health Institute – Lecturer. Baxter – Consultant, Lecturer. Biocodex – Lecturer. BMS – Consultant. Danone – Consultant. Dr. Falk Pharma – Consultant, Lecturer. Fresenius Kabi – Lecturer. Ipsen, Menarini – Lecturer. MyHealth – Grant/Research Support, Lecturer. North Sea Therapeutics – Consultant. Remedus – Lecturer. Takeda Pharmaceuticals – Consultant, Grant/Research Support, Lecturer. Truvion – Consultant, Lecturer. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Consultant, Grant/Research Support, Lecturer.
Simon Lal: B Braun – Honoraria and/or educational support. Baxter – Grant/Research Support, Honoraria and/or educational support. Fresenius Kabi – Grant/Research Support, Honoraria and/or educational support. Northsea – Honoraria and/or educational support. Takeda Pharmaceuticals – Grant/Research Support, Honoraria and/or educational support. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Honoraria and/or educational support. Zealand Pharma – Honoraria and/or educational support.
Kelly Tappenden: Abbott Nutrition Health Institute – Advisor or Review Panel Member, Advisory Committee/Board Member. Nutricia North America – Advisor or Review Panel Member, Advisory Committee/Board Member. OWYN – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Donald Kirby: OWYN – Advisor or Review Panel Member, Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Consultant.
Palle Jeppesen: Albumedix A/S – Honoraria, educational and/or grants. ArTara Therapeutics – Honoraria, educational and/or grants. Bainan Biotech – Honoraria, educational and/or grants. Baxter – Honoraria, educational and/or grants. Coloplast A/S – Honoraria, educational and/or grants. Ferring Pharmaceuticals – Honoraria, educational and/or grants. Fresenius Kabi – Honoraria, educational and/or grants. GlyPharma Therapeutic – Honoraria, educational and/or grants. Hanmi Pharmaceuticals – Honoraria, educational and/or grants. Ironwood Pharmaceuticals – Honoraria, educational and/or grants. Naia Pharmaceuticals – Honoraria, educational and/or grants. NPS Pharmaceuticals – Honoraria, educational and/or grants. Protara Therapeutics – Honoraria, educational and/or grants. Shire pharmaceuticals – Honoraria, educational and/or grants. Takeda pharmaceuticals – Honoraria, educational and/or grants. The Novo Nordisk Foundation – Honoraria, educational and/or grants. Therachon – Honoraria, educational and/or grants. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Honoraria, educational and/or grants. Zealand Pharma – Honoraria, educational and/or grants.
Francisca Joly: Baxter – Grant/Research Support, Lecturer. BBraun – Lecturer. Carembouche – Advisor or Review Panel Member, Consultant, Grant/Research Support. Fresenius Kabi – Lecturer. Hanmi Pharmaceuticals – Advisor or Review Panel Member, Consultant. Ironwood Pharmaceuticals – Advisor or Review Panel Member, Consultant. Janssen and Janssen – Lecturer. Mobile3esolutions – Advisor or Review Panel Member, Consultant, Grant/Research Support. NorthSea Therapeutics – Advisor or Review Panel Member, Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support. Theradial – Lecturer. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Consultant, Grant/Research Support. Zealand Pharma – Advisor or Review Panel Member, Consultant, Grant/Research Support.
Tomasz Masior: Ironwood Pharmaceuticals – Employee.
Patricia Valencia: Ironwood Pharmaceuticals – Employee.
Chang Ming: Ironwood Pharmaceuticals – Employee.
Mena Boules: Ironwood Pharmaceuticals – Employee, Stock-publicly held company(excluding mutual/index funds).
Susanna Huh: Ironwood Pharmaceuticals – Employee.
Kishore Iyer: Hanmi Pharmaceuticals – Advisor or Review Panel Member. Northsea Therapeutics – Advisor or Review Panel Member, Grant/Research Support. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. VectivBio – now part of Ironwood Pharmaceuticals, Inc – Advisor or Review Panel Member, Grant/Research Support.
Tim Vanuytsel, MD, PhD1, Simon Lal, MD, PhD2, Kelly Tappenden, PhD, RD3, Donald Kirby, MD4, Palle Jeppesen, MD, PhD5, Francisca Joly, MD, PhD6, Tomasz Masior, MD7, Patricia Valencia, PharmD7, Chang Ming, MS, PhD7, Mena Boules, MD8, Susanna Huh, MD, MPH7, Kishore Iyer, MD, MBBS9. P1521 - Safety and Tolerability of Once-Weekly Glucagon-Like Peptide-2 Analog Apraglutide in Patients With Short Bowel Syndrome and Intestinal Failure: Results From a Global, Phase 3, Randomized, Double-Blind Trial, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

