Sunday Poster Session
Category: Small Intestine
P1525 - Development and Validation of a Portable Device for At-Home Hydrogen and Methane Breath Testing
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- LP
Lara Pocock, MSc, BSc
Owlstone Medical
Cambridge, England, United Kingdom
Presenting Author(s)
Riccardo Avvisati, PhD, Joshua Bates, PhD, Hannah Winter, , Madeleine Ball, PhD, Lara Pocock, MSc, BSc, Huw Davies, PhD, Rui Pinto-Lopes, MD, FHEA, Francesco Guagliardo, , Martin Nash, PhD, Paul Scott, PhD, Robert L. McCarthy, , Billy Boyle, MS
Owlstone Medical, Cambridge, England, United Kingdom
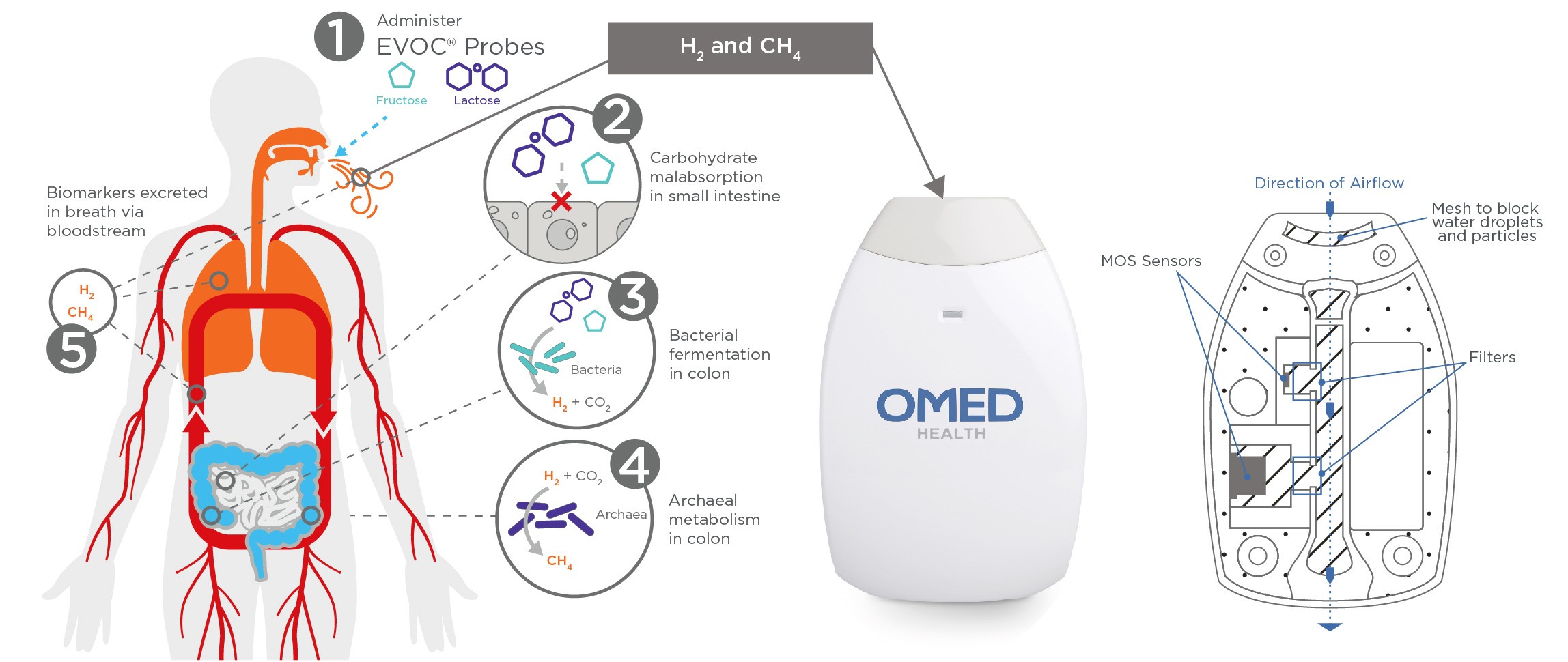
Introduction: Hydrogen and methane breath tests can diagnose small intestinal bacterial overgrowth (SIBO) due to the connection between these gases and the activity of the gut microbiome. We have developed a portable hydrogen and methane breath analyzer device (patent-pending) to support diagnosis, longitudinal monitoring of treatment response and early detection of recurrence. The OMED Health Breath Analyzer utilizes a gas concentration predictive model developed by simulating human breath across a range of temperatures and humidities. We benchmarked the accuracy of the device to an in-clinic HMBT using the breath of volunteers.
Methods: 941 synthetic breath exposures were included in a dataset across clinically relevant concentrations, temperatures, and humidities. The data was split into three datasets (training, validation, and test) to build a gas concentration predictive model. The model was then tested on 243 breath samples collected from 54 participants and compared to the Gastrogenius™ Breath Monitor (HMBT). Each participant gave two samples into the HMBT, and a breath sample into the OMED device. A mean of the two HMBT measurements was calculated (AVG) as the assumed ground truth of breath concentration. The results of the AVG HMBT measurements were then compared with the values from the OMED device for hydrogen and methane.
Results: The sensors in the OMED device and analytical model utilized were shown to assess and report hydrogen and methane levels accurately. Between the device and AVG HMBT, there was a high degree of correspondence between the concentrations detected. The errors (measured as the difference between the OMED device reading and AVG for the OMED device, and the difference between measurements 1 and 2 of HMBT for the HMBT) were comparable for both hydrogen (mean absolute error: OMED: 3.83 ppm, HMBT: 2.11 ppm) and methane (mean absolute error: OMED: 2.32 ppm, HMBT: 1.60 ppm).
Discussion: The OMED device is highly accurate with comparable performance to an in-clinic HMBT. The added portability for convenience and at-home measurements can benefit users by providing longitudinal data immediately available to their healthcare provider.
Disclosures:
Riccardo Avvisati, PhD, Joshua Bates, PhD, Hannah Winter, , Madeleine Ball, PhD, Lara Pocock, MSc, BSc, Huw Davies, PhD, Rui Pinto-Lopes, MD, FHEA, Francesco Guagliardo, , Martin Nash, PhD, Paul Scott, PhD, Robert L. McCarthy, , Billy Boyle, MS. P1525 - Development and Validation of a Portable Device for At-Home Hydrogen and Methane Breath Testing, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Owlstone Medical, Cambridge, England, United Kingdom
Introduction: Hydrogen and methane breath tests can diagnose small intestinal bacterial overgrowth (SIBO) due to the connection between these gases and the activity of the gut microbiome. We have developed a portable hydrogen and methane breath analyzer device (patent-pending) to support diagnosis, longitudinal monitoring of treatment response and early detection of recurrence. The OMED Health Breath Analyzer utilizes a gas concentration predictive model developed by simulating human breath across a range of temperatures and humidities. We benchmarked the accuracy of the device to an in-clinic HMBT using the breath of volunteers.
Methods: 941 synthetic breath exposures were included in a dataset across clinically relevant concentrations, temperatures, and humidities. The data was split into three datasets (training, validation, and test) to build a gas concentration predictive model. The model was then tested on 243 breath samples collected from 54 participants and compared to the Gastrogenius™ Breath Monitor (HMBT). Each participant gave two samples into the HMBT, and a breath sample into the OMED device. A mean of the two HMBT measurements was calculated (AVG) as the assumed ground truth of breath concentration. The results of the AVG HMBT measurements were then compared with the values from the OMED device for hydrogen and methane.
Results: The sensors in the OMED device and analytical model utilized were shown to assess and report hydrogen and methane levels accurately. Between the device and AVG HMBT, there was a high degree of correspondence between the concentrations detected. The errors (measured as the difference between the OMED device reading and AVG for the OMED device, and the difference between measurements 1 and 2 of HMBT for the HMBT) were comparable for both hydrogen (mean absolute error: OMED: 3.83 ppm, HMBT: 2.11 ppm) and methane (mean absolute error: OMED: 2.32 ppm, HMBT: 1.60 ppm).
Discussion: The OMED device is highly accurate with comparable performance to an in-clinic HMBT. The added portability for convenience and at-home measurements can benefit users by providing longitudinal data immediately available to their healthcare provider.

Figure: The OMED Health device uses MOS sensor-based technology to analyze the levels of hydrogen and methane in the breath (patent pending). A diffusion membrane acts as a filter between the main flow path and the sensor chamber to negate the impact of interferant breath VOCs. A mesh (PTFE membrane) prevents moisture from condensing on the sensors and impacting the sensor baseline.
Disclosures:
Riccardo Avvisati indicated no relevant financial relationships.
Joshua Bates indicated no relevant financial relationships.
Hannah Winter indicated no relevant financial relationships.
Madeleine Ball indicated no relevant financial relationships.
Lara Pocock indicated no relevant financial relationships.
Huw Davies indicated no relevant financial relationships.
Rui Pinto-Lopes indicated no relevant financial relationships.
Francesco Guagliardo indicated no relevant financial relationships.
Martin Nash indicated no relevant financial relationships.
Paul Scott indicated no relevant financial relationships.
Robert McCarthy indicated no relevant financial relationships.
Billy Boyle indicated no relevant financial relationships.
Riccardo Avvisati, PhD, Joshua Bates, PhD, Hannah Winter, , Madeleine Ball, PhD, Lara Pocock, MSc, BSc, Huw Davies, PhD, Rui Pinto-Lopes, MD, FHEA, Francesco Guagliardo, , Martin Nash, PhD, Paul Scott, PhD, Robert L. McCarthy, , Billy Boyle, MS. P1525 - Development and Validation of a Portable Device for At-Home Hydrogen and Methane Breath Testing, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
