Sunday Poster Session
Category: Small Intestine
P1528 - Achieving Organoleptic Acceptability with Elemental Formula: A Prospective Single-Blind Comparative Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Ali Rezaie, MD
Cedars-Sinai Medical Center
West Hollywood, CA
Presenting Author(s)
Award: ACG Outstanding Research Award in the Small Intestine Category
Award: Presidential Poster Award
Ali Rezaie, MD1, Bianca W. Chang, MD2, Krystyna Houser, 3, Ava Hosseini, MPH1, Daniel H. Brimberry, PhD2, Mohamad Rashid, MD1, Ruchi Mathur, MD1, Sepideh Mehravar, MD1, Yin Chan, MD2, Gabriela Leite, PhD1, Walter Morales, 1, Maritza Sanchez, 1, Coka Yip, NP2, Stacy Weitsman, MS1, Cristina M. Fajardo, MSc1, Ignacio Rivera, 1, Jiajing Wang, PhD4, Gillian M. Barlow, PhD1, Jason Nasser, MD2, Amrit K.. Kamboj, MD2, Jane Lim, MD2, Christine L.. Scarcello, MS, RD2, Mark Pimentel, MD1
1Cedars-Sinai Medical Center, West Hollywood, CA; 2Cedars-Sinai Medical Center, Los Angeles, CA; 3mBiota, Santa Monica, CA; 4Cedars-Sinai Medical Center, San Jose, CA
Introduction: Elemental diets (EDs) are complete meal replacements containing the required daily allowance of vitamins, major/trace minerals, fat, amino acids, and carbohydrates. EDs have desirable safety and efficacy profiles in several clinical settings including but not limited to Crohn’s disease, eosinophilic esophagitis/gastroenteritis, and small intestinal bacterial overgrowth (SIBO). Palatability remains the main barrier to using complete EDs in clinical practice and results in significant poor compliance in pediatric and adult populations. In this prospective trial, we aimed to blindly assess the organoleptic acceptability of a novel palatable elemental diet (PED) in comparison to a commercially-available elemental diet (CED).
Methods: Adult subjects with intestinal methanogen overgrowth or SIBO undergoing two weeks of exclusive PED (mBiota Elemental, USA) were recruited (NCT05978973). On the initial visit, participants blindly tasted and assessed PED and Vivonex Plus (Nestle, USA) (CED). Formulations were prepared as per manufacturer’s recommendations. Order of tasting was randomly assigned. Participants were asked about their preference between the two formulas and rated the organoleptic characteristics (appearance, smell, taste, aftertaste, and consistency) along with their overall impression of each formulation using a 7-point Likert scale: 1) Like a lot, 2) Like moderately, 3) Like a little, 4) Neither like nor dislike, 5) Dislike a little, 6) Dislike moderately, 7) Dislike a lot. McNemar’s test was used for comparison of paired nominal data.
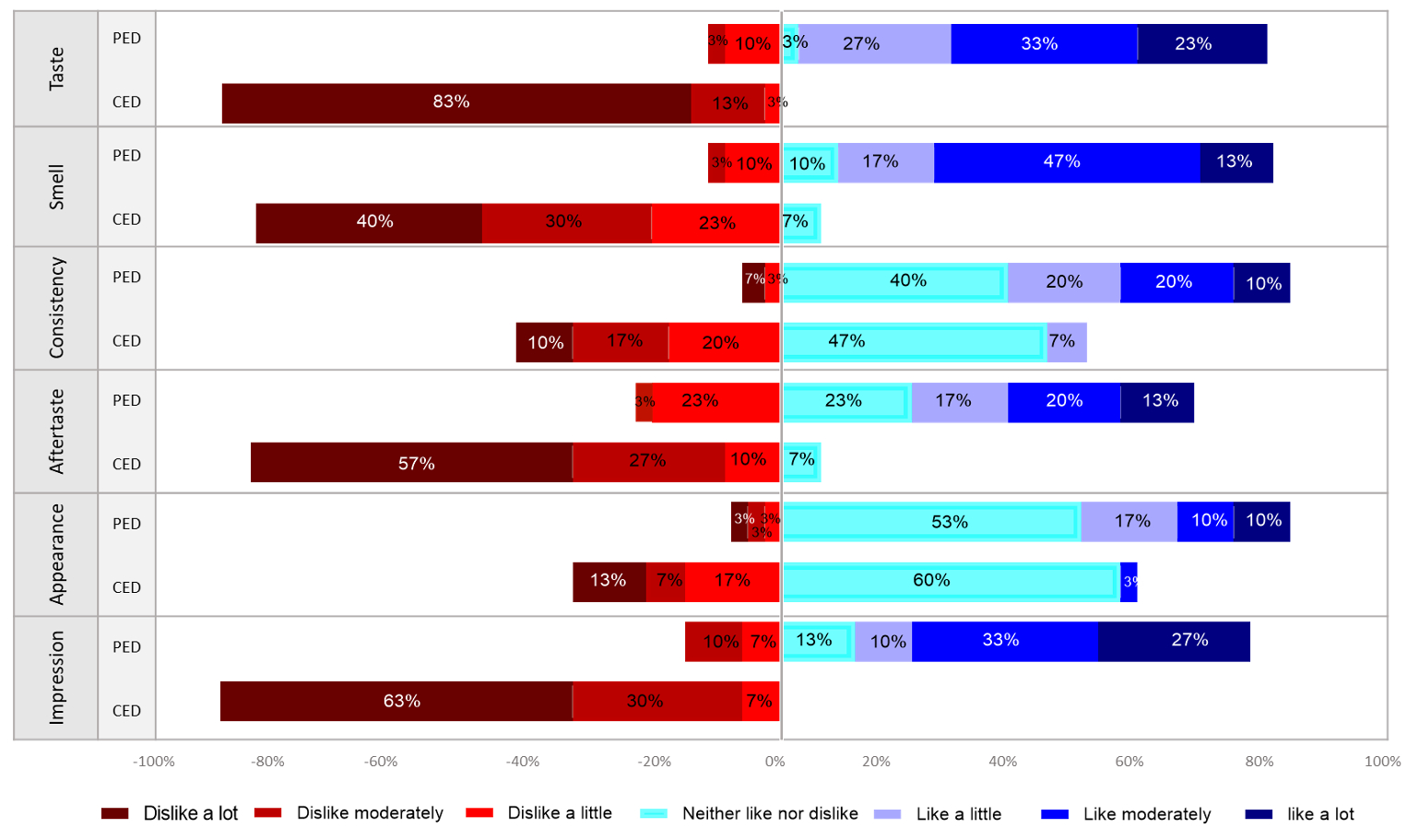
Results: A total of 30 subjects were included (63% women, median (range) age 45 (23-73) years). When asked “Which, if either, of these two shake products tastes better?”, all 30 subjects (100%) preferred PED over CED. As compared to CED, the proportion of subjects who liked or were neutral to the PED (Likert scale 1-4) was significantly higher for all organoleptic characteristics: Appearance (90% vs 63.3%, p=0.027), smell (86.7% vs 6.7%, p< 0.001), taste (86.7% vs 0%, p< 0.001), aftertaste (73.3% vs 6.7%, p< 0.001), consistency (90% vs 53.3%, p=0.006), and overall impression (83.3% vs 0%, p< 0.001) (Figure 1). All 30 subjects (100%) completed the 2-week treatment regimen with exclusive PED.
Discussion: In this prospective, single-blind comparative study, a novel palatable ED formulation was organoleptically more acceptable compared to a conventional ED and led to optimal compliance during a 2-week exclusive ED therapy.
Disclosures:
Ali Rezaie, MD1, Bianca W. Chang, MD2, Krystyna Houser, 3, Ava Hosseini, MPH1, Daniel H. Brimberry, PhD2, Mohamad Rashid, MD1, Ruchi Mathur, MD1, Sepideh Mehravar, MD1, Yin Chan, MD2, Gabriela Leite, PhD1, Walter Morales, 1, Maritza Sanchez, 1, Coka Yip, NP2, Stacy Weitsman, MS1, Cristina M. Fajardo, MSc1, Ignacio Rivera, 1, Jiajing Wang, PhD4, Gillian M. Barlow, PhD1, Jason Nasser, MD2, Amrit K.. Kamboj, MD2, Jane Lim, MD2, Christine L.. Scarcello, MS, RD2, Mark Pimentel, MD1. P1528 - Achieving Organoleptic Acceptability with Elemental Formula: A Prospective Single-Blind Comparative Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Award: Presidential Poster Award
Ali Rezaie, MD1, Bianca W. Chang, MD2, Krystyna Houser, 3, Ava Hosseini, MPH1, Daniel H. Brimberry, PhD2, Mohamad Rashid, MD1, Ruchi Mathur, MD1, Sepideh Mehravar, MD1, Yin Chan, MD2, Gabriela Leite, PhD1, Walter Morales, 1, Maritza Sanchez, 1, Coka Yip, NP2, Stacy Weitsman, MS1, Cristina M. Fajardo, MSc1, Ignacio Rivera, 1, Jiajing Wang, PhD4, Gillian M. Barlow, PhD1, Jason Nasser, MD2, Amrit K.. Kamboj, MD2, Jane Lim, MD2, Christine L.. Scarcello, MS, RD2, Mark Pimentel, MD1
1Cedars-Sinai Medical Center, West Hollywood, CA; 2Cedars-Sinai Medical Center, Los Angeles, CA; 3mBiota, Santa Monica, CA; 4Cedars-Sinai Medical Center, San Jose, CA
Introduction: Elemental diets (EDs) are complete meal replacements containing the required daily allowance of vitamins, major/trace minerals, fat, amino acids, and carbohydrates. EDs have desirable safety and efficacy profiles in several clinical settings including but not limited to Crohn’s disease, eosinophilic esophagitis/gastroenteritis, and small intestinal bacterial overgrowth (SIBO). Palatability remains the main barrier to using complete EDs in clinical practice and results in significant poor compliance in pediatric and adult populations. In this prospective trial, we aimed to blindly assess the organoleptic acceptability of a novel palatable elemental diet (PED) in comparison to a commercially-available elemental diet (CED).
Methods: Adult subjects with intestinal methanogen overgrowth or SIBO undergoing two weeks of exclusive PED (mBiota Elemental, USA) were recruited (NCT05978973). On the initial visit, participants blindly tasted and assessed PED and Vivonex Plus (Nestle, USA) (CED). Formulations were prepared as per manufacturer’s recommendations. Order of tasting was randomly assigned. Participants were asked about their preference between the two formulas and rated the organoleptic characteristics (appearance, smell, taste, aftertaste, and consistency) along with their overall impression of each formulation using a 7-point Likert scale: 1) Like a lot, 2) Like moderately, 3) Like a little, 4) Neither like nor dislike, 5) Dislike a little, 6) Dislike moderately, 7) Dislike a lot. McNemar’s test was used for comparison of paired nominal data.
Results: A total of 30 subjects were included (63% women, median (range) age 45 (23-73) years). When asked “Which, if either, of these two shake products tastes better?”, all 30 subjects (100%) preferred PED over CED. As compared to CED, the proportion of subjects who liked or were neutral to the PED (Likert scale 1-4) was significantly higher for all organoleptic characteristics: Appearance (90% vs 63.3%, p=0.027), smell (86.7% vs 6.7%, p< 0.001), taste (86.7% vs 0%, p< 0.001), aftertaste (73.3% vs 6.7%, p< 0.001), consistency (90% vs 53.3%, p=0.006), and overall impression (83.3% vs 0%, p< 0.001) (Figure 1). All 30 subjects (100%) completed the 2-week treatment regimen with exclusive PED.
Discussion: In this prospective, single-blind comparative study, a novel palatable ED formulation was organoleptically more acceptable compared to a conventional ED and led to optimal compliance during a 2-week exclusive ED therapy.

Figure: Figure 1. Head-to-head comparison of five organoleptic characteristics along with overall impression of palatable elemental diet (PED) compared to a commercially-available elemental diet (CED) based on a 7-point Likert scale.
Disclosures:
Ali Rezaie: Ardelyx – Consultant. Bausch Health – Consultant, Speakers Bureau. Gemelli Biotech – Stock-privately held company. GoodLFE – Stock-privately held company.
Bianca Chang indicated no relevant financial relationships.
Krystyna Houser: mBiota Elemental – Owner/Ownership Interest.
Ava Hosseini indicated no relevant financial relationships.
Daniel Brimberry indicated no relevant financial relationships.
Mohamad Rashid indicated no relevant financial relationships.
Ruchi Mathur: Gemelli – Stock-privately held company. good lfe – Stock-privately held company.
Sepideh Mehravar indicated no relevant financial relationships.
Yin Chan indicated no relevant financial relationships.
Gabriela Leite indicated no relevant financial relationships.
Walter Morales indicated no relevant financial relationships.
Maritza Sanchez indicated no relevant financial relationships.
Coka Yip indicated no relevant financial relationships.
Stacy Weitsman indicated no relevant financial relationships.
Cristina Fajardo indicated no relevant financial relationships.
Ignacio Rivera indicated no relevant financial relationships.
Jiajing Wang indicated no relevant financial relationships.
Gillian Barlow indicated no relevant financial relationships.
Jason Nasser indicated no relevant financial relationships.
Amrit Kamboj: Castle Biosciences – Consultant.
Jane Lim indicated no relevant financial relationships.
Christine Scarcello indicated no relevant financial relationships.
Mark Pimentel: Cylinder Health – Consultant, Stock Options. Dieta Health – Consultant, Stock Options. Ferring – Consultant. Gemelli Biotech – Advisory Committee/Board Member, Intellectual Property/Patents, Stock-privately held company. GoodLFE – Consultant, Stock-privately held company. Salvo Health – Consultant, Stock Options.
Ali Rezaie, MD1, Bianca W. Chang, MD2, Krystyna Houser, 3, Ava Hosseini, MPH1, Daniel H. Brimberry, PhD2, Mohamad Rashid, MD1, Ruchi Mathur, MD1, Sepideh Mehravar, MD1, Yin Chan, MD2, Gabriela Leite, PhD1, Walter Morales, 1, Maritza Sanchez, 1, Coka Yip, NP2, Stacy Weitsman, MS1, Cristina M. Fajardo, MSc1, Ignacio Rivera, 1, Jiajing Wang, PhD4, Gillian M. Barlow, PhD1, Jason Nasser, MD2, Amrit K.. Kamboj, MD2, Jane Lim, MD2, Christine L.. Scarcello, MS, RD2, Mark Pimentel, MD1. P1528 - Achieving Organoleptic Acceptability with Elemental Formula: A Prospective Single-Blind Comparative Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

