Sunday Poster Session
Category: Liver
P1344 - Nutrafol's Hair Gains and Liver Strains
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio
- YS
Yuvraj Singh, BS
Lake Erie College of Osteopathic Medicine
Antelope, CA
Presenting Author(s)
Jasmine Dugal, DO1, Arpinder Malhi, DO1, Yuvraj Singh, BS2, Mark Hsu, MD1, Ahmad Gill, MD3, Preet Patel, MD1, Kyaw Min Tun, DO1
1Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, NV; 2Lake Erie College of Osteopathic Medicine, Antelope, CA; 3Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, CA
Introduction: Idiosyncratic drug-induced liver damage (DILI) is a common occurrence. It manifests as an asymptomatic increase in liver biochemistry, cholestatic or hepatocellular jaundice, liver failure, or chronic hepatitis. Many drugs are known to be associated with DILI, however, non-FDA approved herbals and supplements are less studied in this regard. Nutrafol is a dietary supplement marketed for hair growth. Supplements like nutrafol often perpetuate liver injury due to unregulated doses of non-FDA approved ingredients and allergens found in them.
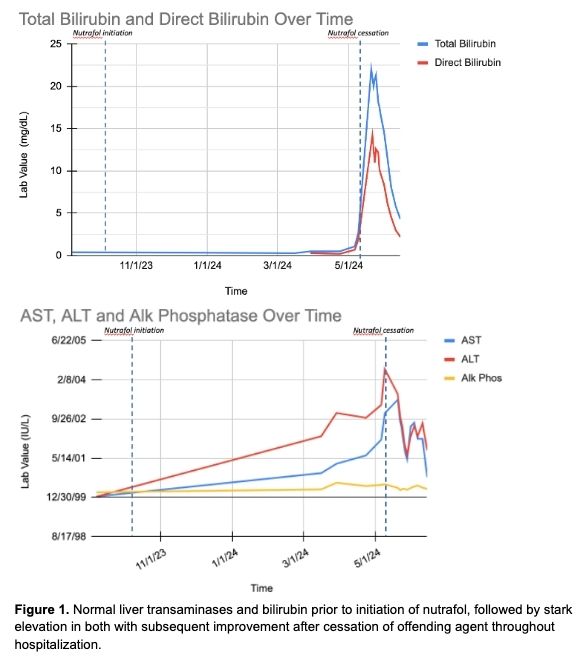
Case Description/Methods: A 26 y/o female with a history of obesity presents with jaundice and an otherwise normal physical exam. Labs on admission were significant for total bilirubin of 22, AST 1247, ALT 1318, and alkaline phosphatase 122, with normal previous liver transaminases (Figure 1). Viral hepatitis, alpha-1 antitrypsin deficiency, CMV, Epstein Barr, anti-mitochondrial ab, soluble liver ag, liver kidney microsomal ab, ANA, anti-smooth muscle, acetaminophen toxicity, serum EtOH, and hereditary hemochromatosis was all negative. She was found to have elevated iron studies, likely secondary to acute hepatitis, elevation in CA 19-9 likely due to cholestasis, elevated serum immunoglobulins without eosinophilia, and multiple food & environmental allergies. CT abdomen, abdominal ultrasound with Doppler, and MRCP were negative for primary hepatic, biliary, or vascular pathologies. Liver biopsy revealed moderate portal inflammation with marked necroinflammatory injury with parenchymal collapse and cholestasis. She denies significant EtOH or hepatotoxic medication use. She reports drinking mullerian leaf tea and initiating a non-FDA approved supplement for hair growth called Nutrafol at the recommendation of a dermatologist five months prior to her presentation. This timeline correlates closely with hyperbilirubinemia and elevation in transaminases (Figure 1).
Discussion: Nutrafol consists of multiple ingredients of which some have potential for hepatotoxicity including turmeric (Grade B), ashwaganda and horsetail (Grade C), saw palmetto (Grade D), kelp minerals and resveratrol (Grade E). Given normal transaminases and bilirubin level one month prior to initiation of Nutrafol followed by development of jaundice with stark elevation in transaminases and bilirubin five months after Nutrafol use, with subsequent improvement in transaminases and eventually bilirubin after cessation of Nutrafol, it is likely that Nutrafol is the cause of DILI in this patient.
Disclosures:
Jasmine Dugal, DO1, Arpinder Malhi, DO1, Yuvraj Singh, BS2, Mark Hsu, MD1, Ahmad Gill, MD3, Preet Patel, MD1, Kyaw Min Tun, DO1. P1344 - Nutrafol's Hair Gains and Liver Strains, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, NV; 2Lake Erie College of Osteopathic Medicine, Antelope, CA; 3Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, CA
Introduction: Idiosyncratic drug-induced liver damage (DILI) is a common occurrence. It manifests as an asymptomatic increase in liver biochemistry, cholestatic or hepatocellular jaundice, liver failure, or chronic hepatitis. Many drugs are known to be associated with DILI, however, non-FDA approved herbals and supplements are less studied in this regard. Nutrafol is a dietary supplement marketed for hair growth. Supplements like nutrafol often perpetuate liver injury due to unregulated doses of non-FDA approved ingredients and allergens found in them.
Case Description/Methods: A 26 y/o female with a history of obesity presents with jaundice and an otherwise normal physical exam. Labs on admission were significant for total bilirubin of 22, AST 1247, ALT 1318, and alkaline phosphatase 122, with normal previous liver transaminases (Figure 1). Viral hepatitis, alpha-1 antitrypsin deficiency, CMV, Epstein Barr, anti-mitochondrial ab, soluble liver ag, liver kidney microsomal ab, ANA, anti-smooth muscle, acetaminophen toxicity, serum EtOH, and hereditary hemochromatosis was all negative. She was found to have elevated iron studies, likely secondary to acute hepatitis, elevation in CA 19-9 likely due to cholestasis, elevated serum immunoglobulins without eosinophilia, and multiple food & environmental allergies. CT abdomen, abdominal ultrasound with Doppler, and MRCP were negative for primary hepatic, biliary, or vascular pathologies. Liver biopsy revealed moderate portal inflammation with marked necroinflammatory injury with parenchymal collapse and cholestasis. She denies significant EtOH or hepatotoxic medication use. She reports drinking mullerian leaf tea and initiating a non-FDA approved supplement for hair growth called Nutrafol at the recommendation of a dermatologist five months prior to her presentation. This timeline correlates closely with hyperbilirubinemia and elevation in transaminases (Figure 1).
Discussion: Nutrafol consists of multiple ingredients of which some have potential for hepatotoxicity including turmeric (Grade B), ashwaganda and horsetail (Grade C), saw palmetto (Grade D), kelp minerals and resveratrol (Grade E). Given normal transaminases and bilirubin level one month prior to initiation of Nutrafol followed by development of jaundice with stark elevation in transaminases and bilirubin five months after Nutrafol use, with subsequent improvement in transaminases and eventually bilirubin after cessation of Nutrafol, it is likely that Nutrafol is the cause of DILI in this patient.

Figure: Figure 1. Normal liver transaminases and bilirubin prior to initiation of nutrafol, followed by stark elevation in both with subsequent improvement after cessation of offending agent throughout hospitalization.
Disclosures:
Jasmine Dugal indicated no relevant financial relationships.
Arpinder Malhi indicated no relevant financial relationships.
Yuvraj Singh indicated no relevant financial relationships.
Mark Hsu indicated no relevant financial relationships.
Ahmad Gill indicated no relevant financial relationships.
Preet Patel indicated no relevant financial relationships.
Kyaw Min Tun indicated no relevant financial relationships.
Jasmine Dugal, DO1, Arpinder Malhi, DO1, Yuvraj Singh, BS2, Mark Hsu, MD1, Ahmad Gill, MD3, Preet Patel, MD1, Kyaw Min Tun, DO1. P1344 - Nutrafol's Hair Gains and Liver Strains, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
