Sunday Poster Session
Category: Liver
P1235 - Making The Cut: A Retrospective Analysis of Patients with Metabolic-Associated Fatty Liver Disease/Metabolic-Associated Steatohepatitis (MAFLD/MASH) Who Have Undergone Bariatric Surgery
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Lakmal Ekanayake, DO
Wright State University Boonshoft School of Medicine
Centerville, OH
Presenting Author(s)
Lakmal Ekanayake, DO1, Nicholas Johnson, BS2, Shaina Ailawadi, MD3, Anay Hindupur, MD2, Erika Lindner, BS2, Erin L. Currey, MD4, Drew Triplett, DO5
1Wright State University Boonshoft School of Medicine, Centerville, OH; 2Wright State University Boonshoft School of Medicine, Dayton, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 4Wright Patterson Medical Center, Wright-Patterson AFB, OH; 5Dayton Gastroenterology, Dayton, OH
Introduction: Metabolic-associated fatty liver disease and metabolic-associated steatohepatitis (MAFLD/MASH) is the leading cause of chronic liver disease worldwide [1]. Currently, there are no approved medications by the FDA for the treatment of MAFLD/MASH. [2] However, bariatric surgery has been found to have substantial benefits to these patients including improvement in histological features of hepatic fibrosis. In our study, we aimed to investigate the total body weight and changes in hemoglobin A1c post-operatively from patients with MAFLD/MASH undergoing bariatric surgery.
Methods: This was a multi-center retrospective cohort study that considered all patients from January 1, 2008, and December 31, 2021, in Dayton, Ohio who underwent bariatric surgery for the primary cause of MAFLD/MASH cirrhosis. Multivariable logistic regression was used to identify independent risk factors. Inferences will be made at the 0.05 level of significance and analyses were conducted using IBM SPSS Statistics 25.0 [4]. Primary endpoints were changes in mean body weight, body mass index (BMI), and changes in hemoglobin A1c at 24 months.
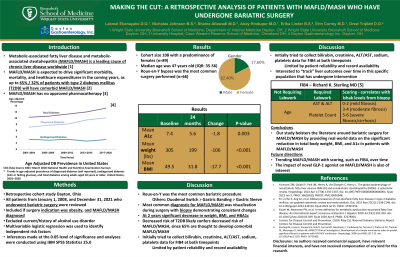
Results: The total cohort size was 108 with a predominance of females (n=89). The median age was 47 years old (IQR: 35-56). Roux-en-Y bypass was the most common surgery performed (n=66). There was a significant difference when comparing the mean A1c at baseline (7.4) and after 24 months (5.6) (p=0.003). There was also a significant difference when comparing mean body weight at baseline (305 pounds) to mean body weight after 24 months (199 pounds) (p< 0.001). Finally, there was a significant difference in mean BMI at baseline (49.5) and after 24 months (31.8) (p< 0.001)
Discussion: In this retrospective cohort study, we assessed the efficacy of bariatric surgery in MAFLD/MASH patients and the effect on total mean body weight, mean BMI, and hemoglobin A1c. Our population saw a significant reduction in hemoglobin A1c at two years in addition to significant reductions in total mean body weight loss. This reiterates the importance of bariatric surgery as an intervention that can modify important risk factors associated with MAFLD/MASH. Our findings are limited by significant loss to bariatric clinic follow-up and lack of pre- and post-bypass liver biopsy. Our study bolsters the literature around bariatric surgery for MAFLD/MASH by providing real-world data on the significant reduction in total body weight, BMI, and A1c in a randomized selection of patients with MAFLD/MASH
Disclosures:
Lakmal Ekanayake, DO1, Nicholas Johnson, BS2, Shaina Ailawadi, MD3, Anay Hindupur, MD2, Erika Lindner, BS2, Erin L. Currey, MD4, Drew Triplett, DO5. P1235 - Making The Cut: A Retrospective Analysis of Patients with Metabolic-Associated Fatty Liver Disease/Metabolic-Associated Steatohepatitis (MAFLD/MASH) Who Have Undergone Bariatric Surgery, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Wright State University Boonshoft School of Medicine, Centerville, OH; 2Wright State University Boonshoft School of Medicine, Dayton, OH; 3University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 4Wright Patterson Medical Center, Wright-Patterson AFB, OH; 5Dayton Gastroenterology, Dayton, OH
Introduction: Metabolic-associated fatty liver disease and metabolic-associated steatohepatitis (MAFLD/MASH) is the leading cause of chronic liver disease worldwide [1]. Currently, there are no approved medications by the FDA for the treatment of MAFLD/MASH. [2] However, bariatric surgery has been found to have substantial benefits to these patients including improvement in histological features of hepatic fibrosis. In our study, we aimed to investigate the total body weight and changes in hemoglobin A1c post-operatively from patients with MAFLD/MASH undergoing bariatric surgery.
Methods: This was a multi-center retrospective cohort study that considered all patients from January 1, 2008, and December 31, 2021, in Dayton, Ohio who underwent bariatric surgery for the primary cause of MAFLD/MASH cirrhosis. Multivariable logistic regression was used to identify independent risk factors. Inferences will be made at the 0.05 level of significance and analyses were conducted using IBM SPSS Statistics 25.0 [4]. Primary endpoints were changes in mean body weight, body mass index (BMI), and changes in hemoglobin A1c at 24 months.
Results: The total cohort size was 108 with a predominance of females (n=89). The median age was 47 years old (IQR: 35-56). Roux-en-Y bypass was the most common surgery performed (n=66). There was a significant difference when comparing the mean A1c at baseline (7.4) and after 24 months (5.6) (p=0.003). There was also a significant difference when comparing mean body weight at baseline (305 pounds) to mean body weight after 24 months (199 pounds) (p< 0.001). Finally, there was a significant difference in mean BMI at baseline (49.5) and after 24 months (31.8) (p< 0.001)
Discussion: In this retrospective cohort study, we assessed the efficacy of bariatric surgery in MAFLD/MASH patients and the effect on total mean body weight, mean BMI, and hemoglobin A1c. Our population saw a significant reduction in hemoglobin A1c at two years in addition to significant reductions in total mean body weight loss. This reiterates the importance of bariatric surgery as an intervention that can modify important risk factors associated with MAFLD/MASH. Our findings are limited by significant loss to bariatric clinic follow-up and lack of pre- and post-bypass liver biopsy. Our study bolsters the literature around bariatric surgery for MAFLD/MASH by providing real-world data on the significant reduction in total body weight, BMI, and A1c in a randomized selection of patients with MAFLD/MASH
Disclosures:
Lakmal Ekanayake indicated no relevant financial relationships.
Nicholas Johnson indicated no relevant financial relationships.
Shaina Ailawadi indicated no relevant financial relationships.
Anay Hindupur indicated no relevant financial relationships.
Erika Lindner indicated no relevant financial relationships.
Erin Currey indicated no relevant financial relationships.
Drew Triplett indicated no relevant financial relationships.
Lakmal Ekanayake, DO1, Nicholas Johnson, BS2, Shaina Ailawadi, MD3, Anay Hindupur, MD2, Erika Lindner, BS2, Erin L. Currey, MD4, Drew Triplett, DO5. P1235 - Making The Cut: A Retrospective Analysis of Patients with Metabolic-Associated Fatty Liver Disease/Metabolic-Associated Steatohepatitis (MAFLD/MASH) Who Have Undergone Bariatric Surgery, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
