Sunday Poster Session
Category: Liver
P1182 - Risk of Hepatotoxicity With Long-Term Methotrexate Use: A Systematic Review and Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Fariha Hasan, MD
Cooper University Hospital
Philadelphia, PA
Presenting Author(s)
Fariha Hasan, MD1, Muhammad Shahzil, MD2, Farhan Naeem, MD3, Ayesha Liaquat, MBBS4, Alexander Garcia, DO5, Avneet Singh, DO5, Muhammad YN. Chaudhary, MBChB6, Andrew Alabd, MD5, Rachel Frank, MD7
1Cooper University Hospital, Philadelphia, PA; 2Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Dow Medical College, Karachi, Sindh, Pakistan; 5Cooper University Hospital, Camden, NJ; 6Indiana University Southwest, Evansville, IN; 7Cooper University Hospital, Mt. Laurel, NJ
Introduction: Methotrexate (MTX) is a commonly used anti-metabolite for the treatment of Inflammatory Bowel Diseases (IBD) and autoimmune diseases such as rheumatoid arthritis (RA) and psoriasis. It has been debated for its potential hepatotoxic effects following long-term use, with multiple guidelines recommending strict serologic monitoring as well as liver biopsies for signs of liver injury. This is the first meta-analysis assessing the risk of hepatotoxicity in patients undergoing prolonged methotrexate therapy compared to a non-methotrexate (non-MTX) group.
Methods: PubMed, Embase, Web of Science, and the Cochrane Library databases were searched for studies comparing liver injury markers between MTX and non-MTX groups. Standard meta-analysis methods were employed using the random-effects model. Generic inverse variance method was used to address clinical heterogeneity. Results were considered significant at p < 0.05, and outcomes were reported as odds ratios and mean differences.
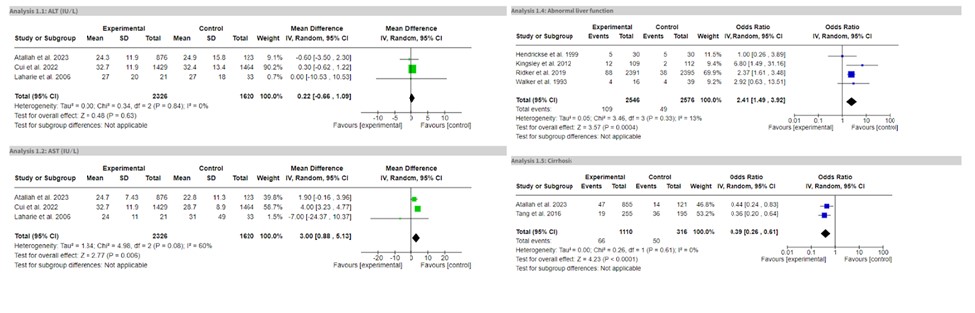
Results: Eleven studies comprising 5,863 patients were included. The MTX group showed a significant increase in liver enzymes (OR = 2.41, 95% CI: 1.49-3.92, P = 0.0004). There was a significant increase in AST levels (MD = 3.00, 95% CI: 0.88-5.13, P = 0.006) compared to the non-MTX group. However, prolonged MTX use did not result in significantly elevated ALT levels (MD = 0.22, 95% CI: -0.66-1.09, P = 0.63), liver fibrosis (MD = -0.01, 95% CI: -0.04-0.02, P = 0.64), or adverse events (OR = 1.73, 95% CI: 0.40-7.42, P = 0.46) such as nausea, vomiting, shortness of breath, recurrent infections etc. Similarly, no significantly increased risk of cirrhosis (OR = 0.39, 95% CI: 0.26-0.61, P < 0.0001) was observed with long-term MTX use.
Discussion: This meta-analysis challenges the widely accepted favorable safety profile of long-term MTX use, suggesting that while certain liver enzymes such as AST may be elevated, the overall risk of severe hepatotoxic outcomes, including cirrhosis and liver fibrosis, remains low. This highlights the importance of individualized patient monitoring and suggests that routine liver biopsies may not be necessary for all patients on long-term MTX therapy. However, the significant risk of abnormal liver function following prolonged MTX therapy warrants caution. These findings highlight the need for further research to explore this correlation in depth. Additional randomized controlled trials are essential to determine the hepatotoxic effects of MTX use and refine monitoring guidelines.
Disclosures:
Fariha Hasan, MD1, Muhammad Shahzil, MD2, Farhan Naeem, MD3, Ayesha Liaquat, MBBS4, Alexander Garcia, DO5, Avneet Singh, DO5, Muhammad YN. Chaudhary, MBChB6, Andrew Alabd, MD5, Rachel Frank, MD7. P1182 - Risk of Hepatotoxicity With Long-Term Methotrexate Use: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Cooper University Hospital, Philadelphia, PA; 2Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4Dow Medical College, Karachi, Sindh, Pakistan; 5Cooper University Hospital, Camden, NJ; 6Indiana University Southwest, Evansville, IN; 7Cooper University Hospital, Mt. Laurel, NJ
Introduction: Methotrexate (MTX) is a commonly used anti-metabolite for the treatment of Inflammatory Bowel Diseases (IBD) and autoimmune diseases such as rheumatoid arthritis (RA) and psoriasis. It has been debated for its potential hepatotoxic effects following long-term use, with multiple guidelines recommending strict serologic monitoring as well as liver biopsies for signs of liver injury. This is the first meta-analysis assessing the risk of hepatotoxicity in patients undergoing prolonged methotrexate therapy compared to a non-methotrexate (non-MTX) group.
Methods: PubMed, Embase, Web of Science, and the Cochrane Library databases were searched for studies comparing liver injury markers between MTX and non-MTX groups. Standard meta-analysis methods were employed using the random-effects model. Generic inverse variance method was used to address clinical heterogeneity. Results were considered significant at p < 0.05, and outcomes were reported as odds ratios and mean differences.
Results: Eleven studies comprising 5,863 patients were included. The MTX group showed a significant increase in liver enzymes (OR = 2.41, 95% CI: 1.49-3.92, P = 0.0004). There was a significant increase in AST levels (MD = 3.00, 95% CI: 0.88-5.13, P = 0.006) compared to the non-MTX group. However, prolonged MTX use did not result in significantly elevated ALT levels (MD = 0.22, 95% CI: -0.66-1.09, P = 0.63), liver fibrosis (MD = -0.01, 95% CI: -0.04-0.02, P = 0.64), or adverse events (OR = 1.73, 95% CI: 0.40-7.42, P = 0.46) such as nausea, vomiting, shortness of breath, recurrent infections etc. Similarly, no significantly increased risk of cirrhosis (OR = 0.39, 95% CI: 0.26-0.61, P < 0.0001) was observed with long-term MTX use.
Discussion: This meta-analysis challenges the widely accepted favorable safety profile of long-term MTX use, suggesting that while certain liver enzymes such as AST may be elevated, the overall risk of severe hepatotoxic outcomes, including cirrhosis and liver fibrosis, remains low. This highlights the importance of individualized patient monitoring and suggests that routine liver biopsies may not be necessary for all patients on long-term MTX therapy. However, the significant risk of abnormal liver function following prolonged MTX therapy warrants caution. These findings highlight the need for further research to explore this correlation in depth. Additional randomized controlled trials are essential to determine the hepatotoxic effects of MTX use and refine monitoring guidelines.

Figure: Forest plots for outcomes-ALT level, AST level, Abnormal Liver Function Test and Cirrhosis
Disclosures:
Fariha Hasan indicated no relevant financial relationships.
Muhammad Shahzil indicated no relevant financial relationships.
Farhan Naeem indicated no relevant financial relationships.
Ayesha Liaquat indicated no relevant financial relationships.
Alexander Garcia indicated no relevant financial relationships.
Avneet Singh indicated no relevant financial relationships.
Muhammad Chaudhary indicated no relevant financial relationships.
Andrew Alabd indicated no relevant financial relationships.
Rachel Frank indicated no relevant financial relationships.
Fariha Hasan, MD1, Muhammad Shahzil, MD2, Farhan Naeem, MD3, Ayesha Liaquat, MBBS4, Alexander Garcia, DO5, Avneet Singh, DO5, Muhammad YN. Chaudhary, MBChB6, Andrew Alabd, MD5, Rachel Frank, MD7. P1182 - Risk of Hepatotoxicity With Long-Term Methotrexate Use: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
