Sunday Poster Session
Category: IBD
P0877 - Efficacy of Turmeric (Curcumin) Plus Mesalamine in Ulcerative Colitis: A Propensity-Matched Cohort Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Himsikhar Khataniar, MD
Allegheny General Hospital
Pittsburgh, PA
Presenting Author(s)
Himsikhar Khataniar, MD1, Fjona Tabaku, MD1, Taimur S. Muzammil, MD1, Jana G. Hashash, MD, MSc2, Francis A. Farraye, MD, MSc2, Gursimran S. Kochhar, MD1, Aakash Desai, MD3
1Allegheny General Hospital, Pittsburgh, PA; 2Mayo Clinic, Jacksonville, FL; 3Mayo Clinic, Pittsburgh, PA
Introduction: The American Gastroenterology Association advises initiating a standard dose of mesalamine for patients with extensive mild-to-moderate ulcerative colitis (UC). Several randomized controlled trials have shown the enhanced clinical and endoscopic response from combining curcumin with mesalamine; however, these were limited by sample size and lack of long-term data.
Methods: In this retrospective cohort study, we utilized TriNetX, a comprehensive multi-institutional database, to evaluate the efficacy of combining curcumin with mesalamine compared to mesalamine alone (control cohort) in patients with UC. The primary objective was the risk of oral and/or intravenous (IV) steroid use and escalation to advanced therapy within 12 months. The secondary objective was risk of colectomy, oral steroid use, all cause emergency department (ED) visits and hospitalization. One-to-one propensity score matching (PSM) was performed for demographics, comorbid conditions, laboratory variables (hemoglobin, C-reactive protein, albumin and calprotectin), disease extent and previous steroid use. Risks were quantified as adjusted odds ratios (aOR) with corresponding 95% confidence intervals (CI).
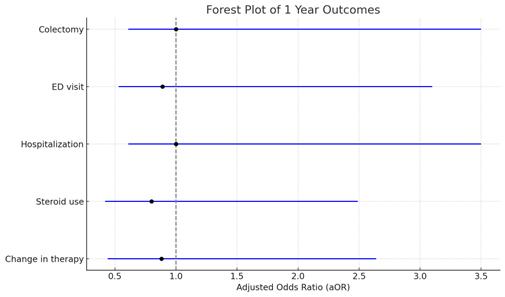
Results: The study included 104 patients in the mesalamine and curcumin cohort (mean age 49.5±17.1 years, 57.69% female, 75% White, 36.6% pancolitis, 28.4% proctitis) and 46,607 patients in the mesalamine-only cohort (mean age 47.5±17.4 years, 50.40% female sex, 71.11% White, 37.9% pancolitis, 26.3% proctitis). PSM revealed no statistically significant differences in the risk of oral and/or IV steroid use (aOR 0.83, CI 0.32-1.69) and advanced therapy use (aOR 0.88, CI 0.44-1.76) within 12 months of treatment initiation. Additionally, there was no difference in the risk for colectomy (aOR 1, CI 0.39-2.51), all cause ED visits (aOR 0.89, CI 0.36-2.21), and all cause hospitalization (aOR 1, CI 0.39-2.51) between the two cohorts.
Discussion: Our findings indicate that, in a real-world setting, the combination of mesalamine and curcumin was not associated with significant differences in clinical outcomes within one year of treatment initiation when compared to mesalamine alone. This warrants further prospective studies with larger sample sizes and long term follow up to validate our findings.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Himsikhar Khataniar, MD1, Fjona Tabaku, MD1, Taimur S. Muzammil, MD1, Jana G. Hashash, MD, MSc2, Francis A. Farraye, MD, MSc2, Gursimran S. Kochhar, MD1, Aakash Desai, MD3. P0877 - Efficacy of Turmeric (Curcumin) Plus Mesalamine in Ulcerative Colitis: A Propensity-Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Allegheny General Hospital, Pittsburgh, PA; 2Mayo Clinic, Jacksonville, FL; 3Mayo Clinic, Pittsburgh, PA
Introduction: The American Gastroenterology Association advises initiating a standard dose of mesalamine for patients with extensive mild-to-moderate ulcerative colitis (UC). Several randomized controlled trials have shown the enhanced clinical and endoscopic response from combining curcumin with mesalamine; however, these were limited by sample size and lack of long-term data.
Methods: In this retrospective cohort study, we utilized TriNetX, a comprehensive multi-institutional database, to evaluate the efficacy of combining curcumin with mesalamine compared to mesalamine alone (control cohort) in patients with UC. The primary objective was the risk of oral and/or intravenous (IV) steroid use and escalation to advanced therapy within 12 months. The secondary objective was risk of colectomy, oral steroid use, all cause emergency department (ED) visits and hospitalization. One-to-one propensity score matching (PSM) was performed for demographics, comorbid conditions, laboratory variables (hemoglobin, C-reactive protein, albumin and calprotectin), disease extent and previous steroid use. Risks were quantified as adjusted odds ratios (aOR) with corresponding 95% confidence intervals (CI).
Results: The study included 104 patients in the mesalamine and curcumin cohort (mean age 49.5±17.1 years, 57.69% female, 75% White, 36.6% pancolitis, 28.4% proctitis) and 46,607 patients in the mesalamine-only cohort (mean age 47.5±17.4 years, 50.40% female sex, 71.11% White, 37.9% pancolitis, 26.3% proctitis). PSM revealed no statistically significant differences in the risk of oral and/or IV steroid use (aOR 0.83, CI 0.32-1.69) and advanced therapy use (aOR 0.88, CI 0.44-1.76) within 12 months of treatment initiation. Additionally, there was no difference in the risk for colectomy (aOR 1, CI 0.39-2.51), all cause ED visits (aOR 0.89, CI 0.36-2.21), and all cause hospitalization (aOR 1, CI 0.39-2.51) between the two cohorts.
Discussion: Our findings indicate that, in a real-world setting, the combination of mesalamine and curcumin was not associated with significant differences in clinical outcomes within one year of treatment initiation when compared to mesalamine alone. This warrants further prospective studies with larger sample sizes and long term follow up to validate our findings.

Figure: Forest Plot depicting 1 year outcomes post Mesalamine plus Curcumin in UC
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Himsikhar Khataniar indicated no relevant financial relationships.
Fjona Tabaku indicated no relevant financial relationships.
Taimur Muzammil indicated no relevant financial relationships.
Jana Hashash: Bristol Myers Squibb – Consultant.
Francis Farraye: AbbVie – Consultant. Avalo Therapeutics – Consultant. Bausch – Advisor or Review Panel Member. BMS – Consultant. Braintree Labs – Consultant. DSMB for Lilly. – Sits on. Fresenius Kabi – Consultant. GI Reviewers and IBD Educational Group – independent contractor. GSK, Iterative Health, Janssen, Pfizer, Pharmacosmos, Sandoz Immunology, Sebela and Viatris – Consultant.
Gursimran Kochhar: Boston Scientific Endoscopy – Consultant. DigbI – Stock Options. Eli Lilli – Advisory Committee/Board Member, Speakers Bureau. Olympus Endoscopy – Consultant. Pentax Endoscopy – Consultant. Takeda – Consultant.
Aakash Desai indicated no relevant financial relationships.
Himsikhar Khataniar, MD1, Fjona Tabaku, MD1, Taimur S. Muzammil, MD1, Jana G. Hashash, MD, MSc2, Francis A. Farraye, MD, MSc2, Gursimran S. Kochhar, MD1, Aakash Desai, MD3. P0877 - Efficacy of Turmeric (Curcumin) Plus Mesalamine in Ulcerative Colitis: A Propensity-Matched Cohort Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
