Sunday Poster Session
Category: IBD
P0887 - C-Reactive Protein/Albumin Ratio: A Reliable Serum Marker for Evaluating Endoscopic Disease Activity in Inflammatory Bowel Diseases
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

S. Sharareh Dehghani, MD
Montefiore Medical Center, Albert Einstein College of Medicine
Bronx, NY
Presenting Author(s)
S. Sharareh Dehghani, MD1, Arunima Kaul, MD1, Carlos Figueredo, MD1, Hemali Rochlani, MD1, Xianhong Xie, PhD2, Ruby Greywoode, MD, MS1, Thomas Ullman, MD1
1Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY; 2Albert Einstein College of Medicine, Bronx, NY
Introduction: Inflammatory Bowel Diseases (IBD), including UC and CD, are chronic conditions characterized by periods of exacerbation and remission.1,2 Although colonoscopy is considered the most accurate method for assessing disease severity during exacerbation episodes, its disadvantages—such as high cost, invasiveness, and potential complications—make it a less favorable option for these patients. 3 In this study, we evaluated the association between CRP/Albumin ratio (CAR) and colonoscopy scores in IBD patients to develop a serum marker-based scoring system for assessing disease severity.
Methods: In this retrospective study, patients with IBD diagnosis and no concurrent infection or malignancy were included and CAR, fecal calprotectin (FC) and CBC values from within one week of the procedure were collected. The correlation between CAR and standard colonoscopy scores (Mayo score for UC and Simple Endoscopic Activity Score for CD) was assessed to evaluate the biomarker’s accuracy in predicting disease severity. After bivariate analyses, the most significant laboratory biomarkers were included in multivariable models for IBD disease severity.
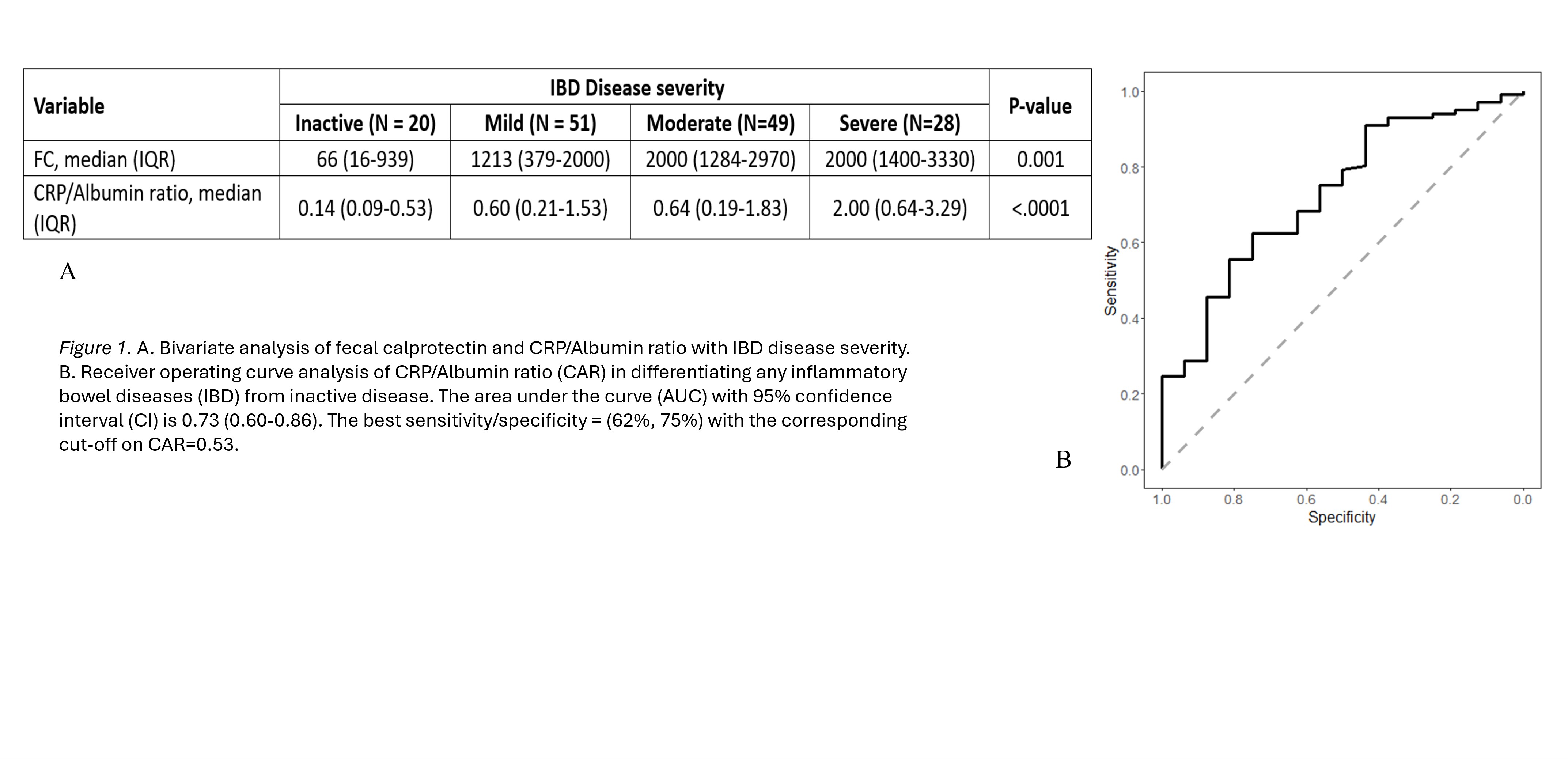
Results: 118 patients and 148 colonoscopies were included in this study. CAR correlated positively with FC level and platelet count and negatively with hemoglobin level. In the bivariate analysis, both FC and CAR were associated with the severity of the disease (Figure 1). A multivariable model identified CAR as a significant predictor of disease severity in both subgroups of UC and CD (P= < 0.0001)
CAR was able to distinguish any IBD disease from inactive disease with area under the receiver operating characteristic curve (AUC) of 0.73 (95% CI of 0.60–0.86) with sensitivity of 62% and specificity of 75% (Figure 1).
Discussion: Interest in replacing colonoscopy with non-invasive serum biomarkers for assessing IBD has grown in recent years due to the complications and high costs associated with endoscopic evaluations and the inconvenience with stool collection.4 This study evaluates the association between CAR and standard colonoscopy scores in IBD patients, demonstrating that CAR can accurately predict disease severity. This study demonstrates that this ratio can replace colonoscopy for assessing endoscopic disease severity during UC and CD exacerbations. We hope this study initiates the development of blood diagnostic and prognostic models for IBD patients, reducing the need for colonoscopy during exacerbations.
Disclosures:
S. Sharareh Dehghani, MD1, Arunima Kaul, MD1, Carlos Figueredo, MD1, Hemali Rochlani, MD1, Xianhong Xie, PhD2, Ruby Greywoode, MD, MS1, Thomas Ullman, MD1. P0887 - C-Reactive Protein/Albumin Ratio: A Reliable Serum Marker for Evaluating Endoscopic Disease Activity in Inflammatory Bowel Diseases, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY; 2Albert Einstein College of Medicine, Bronx, NY
Introduction: Inflammatory Bowel Diseases (IBD), including UC and CD, are chronic conditions characterized by periods of exacerbation and remission.1,2 Although colonoscopy is considered the most accurate method for assessing disease severity during exacerbation episodes, its disadvantages—such as high cost, invasiveness, and potential complications—make it a less favorable option for these patients. 3 In this study, we evaluated the association between CRP/Albumin ratio (CAR) and colonoscopy scores in IBD patients to develop a serum marker-based scoring system for assessing disease severity.
Methods: In this retrospective study, patients with IBD diagnosis and no concurrent infection or malignancy were included and CAR, fecal calprotectin (FC) and CBC values from within one week of the procedure were collected. The correlation between CAR and standard colonoscopy scores (Mayo score for UC and Simple Endoscopic Activity Score for CD) was assessed to evaluate the biomarker’s accuracy in predicting disease severity. After bivariate analyses, the most significant laboratory biomarkers were included in multivariable models for IBD disease severity.
Results: 118 patients and 148 colonoscopies were included in this study. CAR correlated positively with FC level and platelet count and negatively with hemoglobin level. In the bivariate analysis, both FC and CAR were associated with the severity of the disease (Figure 1). A multivariable model identified CAR as a significant predictor of disease severity in both subgroups of UC and CD (P= < 0.0001)
CAR was able to distinguish any IBD disease from inactive disease with area under the receiver operating characteristic curve (AUC) of 0.73 (95% CI of 0.60–0.86) with sensitivity of 62% and specificity of 75% (Figure 1).
Discussion: Interest in replacing colonoscopy with non-invasive serum biomarkers for assessing IBD has grown in recent years due to the complications and high costs associated with endoscopic evaluations and the inconvenience with stool collection.4 This study evaluates the association between CAR and standard colonoscopy scores in IBD patients, demonstrating that CAR can accurately predict disease severity. This study demonstrates that this ratio can replace colonoscopy for assessing endoscopic disease severity during UC and CD exacerbations. We hope this study initiates the development of blood diagnostic and prognostic models for IBD patients, reducing the need for colonoscopy during exacerbations.

Figure: .
Disclosures:
S. Sharareh Dehghani indicated no relevant financial relationships.
Arunima Kaul indicated no relevant financial relationships.
Carlos Figueredo indicated no relevant financial relationships.
Hemali Rochlani indicated no relevant financial relationships.
Xianhong Xie indicated no relevant financial relationships.
Ruby Greywoode: Janssen – Grant/Research Support.
Thomas Ullman: BMS – Consultant. Pfizer – Advisor or Review Panel Member, Grant/Research Support.
S. Sharareh Dehghani, MD1, Arunima Kaul, MD1, Carlos Figueredo, MD1, Hemali Rochlani, MD1, Xianhong Xie, PhD2, Ruby Greywoode, MD, MS1, Thomas Ullman, MD1. P0887 - C-Reactive Protein/Albumin Ratio: A Reliable Serum Marker for Evaluating Endoscopic Disease Activity in Inflammatory Bowel Diseases, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
