Tuesday Poster Session
Category: Functional Bowel Disease
P4047 - Pilot Study of Crofelemer for Functional Diarrhea
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- VR
Vikram Rangan, MD
Beth Israel Deaconess Medical Center, Harvard Medical School
Boston, MA
Presenting Author(s)
Award: Presidential Poster Award
Vikram Rangan, MD1, Colin Wu, BS1, Johanna Iturrino, MD2, Anthony J. Lembo, MD3, Judy Nee, MD1
1Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA; 2BIDMC, Chestnut Hill, MA; 3Digestive Disease Institute, Cleveland Clinic, Cleveland, OH
Introduction: Functional diarrhea (FDr) is a common diagnosis with limited treatments. Crofelemer, a botanical drug purified from the latex of Croton lechleri, is a potent anti-secretory anti-diarrheal, which has been FDA approved for symptomatic relief of HIV/AIDS related diarrhea in adults. This pilot trial evaluated its efficacy and safety in FDr.
Methods: Following a 4 week baseline period, adults with FDr (Rome IV criteria) were randomized to receive crofelemer (125 mg) or placebo orally twice daily for 4 weeks (double blind period) followed by 4 weeks of open label crofelemer (open label period). Patients reported daily overall Bristol Stool Form Score (BSFS), number of bowel movements, worst abdominal pain score (0-10 numeric rating scale), and abdominal discomfort score. The primary endpoint was change in mean daily BSFS between baseline and double blind period.
Results:17 FDr patients completed the 4 week double blind period (crofelemer n=9, placebo=8). Average BSFS decreased from 5.88 +/- 0.35 (mean +/- SD) at baseline to 5.28 +/- 0.66 in the crofelemer group. Average BSFS decreased from 6.09 +/- 0.60 to 5.85 +/- 0.74 in the placebo group. The difference in change score of BSFS between crofelemer and placebo groups approached statistical significance (-0.60 +/- 0.50 vs -0.24 +/- 0.32, respectively; 1 sided p value 0.056). The mean worst abdominal pain decreased significantly in the crofelemer group compared to placebo group (57.0% vs. 12.4%, respectively p=0.002).
Vikram Rangan, MD1, Colin Wu, BS1, Johanna Iturrino, MD2, Anthony J. Lembo, MD3, Judy Nee, MD1
1Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA; 2BIDMC, Chestnut Hill, MA; 3Digestive Disease Institute, Cleveland Clinic, Cleveland, OH
Introduction: Functional diarrhea (FDr) is a common diagnosis with limited treatments. Crofelemer, a botanical drug purified from the latex of Croton lechleri, is a potent anti-secretory anti-diarrheal, which has been FDA approved for symptomatic relief of HIV/AIDS related diarrhea in adults. This pilot trial evaluated its efficacy and safety in FDr.
Methods: Following a 4 week baseline period, adults with FDr (Rome IV criteria) were randomized to receive crofelemer (125 mg) or placebo orally twice daily for 4 weeks (double blind period) followed by 4 weeks of open label crofelemer (open label period). Patients reported daily overall Bristol Stool Form Score (BSFS), number of bowel movements, worst abdominal pain score (0-10 numeric rating scale), and abdominal discomfort score. The primary endpoint was change in mean daily BSFS between baseline and double blind period.
Results:
17 FDr patients completed the 4 week double blind period (crofelemer n=9, placebo=8). Average BSFS decreased from 5.88 +/- 0.35 (mean +/- SD) at baseline to 5.28 +/- 0.66 in the crofelemer group. Average BSFS decreased from 6.09 +/- 0.60 to 5.85 +/- 0.74 in the placebo group. The difference in change score of BSFS between crofelemer and placebo groups approached statistical significance (-0.60 +/- 0.50 vs -0.24 +/- 0.32, respectively; 1 sided p value 0.056). The mean worst abdominal pain decreased significantly in the crofelemer group compared to placebo group (57.0% vs. 12.4%, respectively p=0.002).
A total of 14 patients continued on open label crofelemer for an additional 4 weeks (8 in crofelemer, 6 in placebo). Among the 8 patients who continued to receive open label crofelemer, mean BSFS decreased from 5.28 +/- 0.66 to 4.75 +/- 0.55. Overall change in BSFS compared to baseline was -1.12 +/- -0.54, or -59.7%. Among patients who crossed over from placebo to open label crofelemer, mean BSFS decreased from 5.85 +/- 0.74 to 5.57 +/- 0.98. Overall change in BSFS compared to baseline was -0.50 +/- -0.42, or -29.5%. Crofelemer was well-tolerated with a side effect profile similar to placebo, without serious adverse events.
Discussion: In this pilot study, crofelemer decreased stool consistency and abdominal pain without significant side effects, including constipation. Crofelemer appears to be safe and effective in FDr, and may thus be a particularly practical option in those with history of fluctuating stool consistency. Larger trials are needed to more thoroughly assess safety and longer term efficacy.
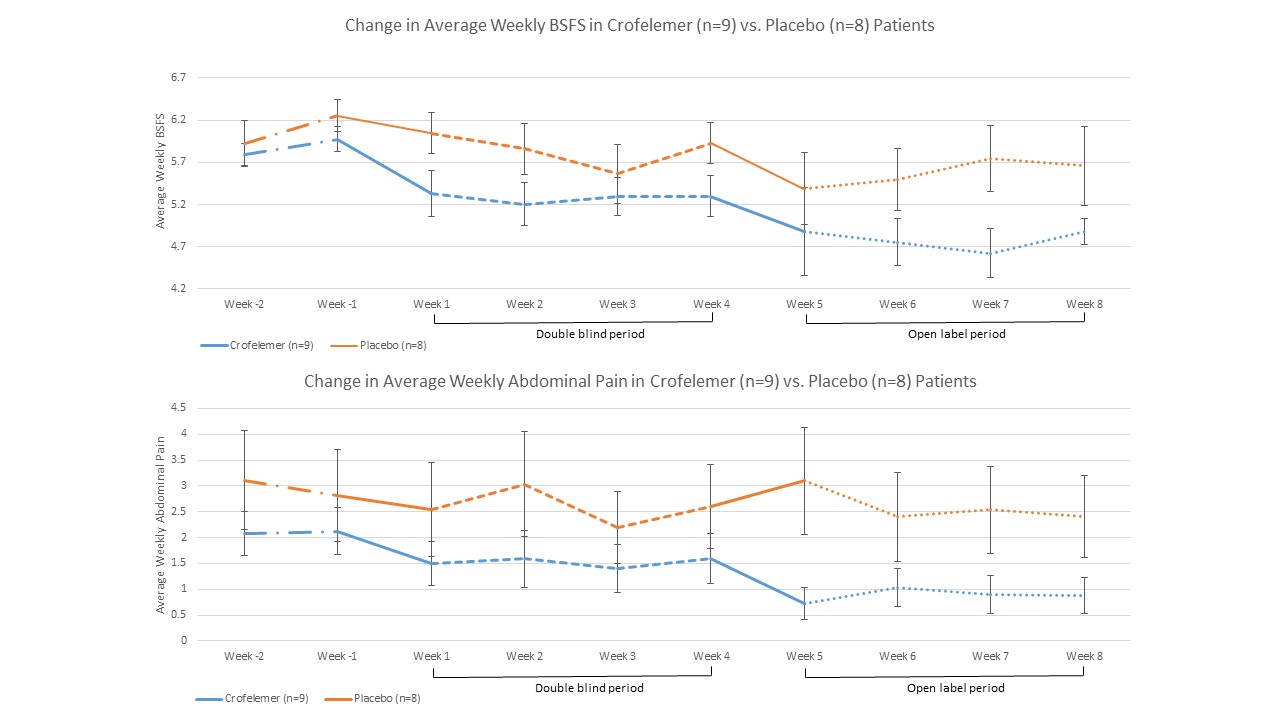
Figure: Change in BSFS and abdominal pain
Disclosures:
Vikram Rangan indicated no relevant financial relationships. Colin Wu indicated no relevant financial relationships. Johanna Iturrino indicated no relevant financial relationships. Anthony Lembo: Allurion – Stock Options. Bristol Myers Squibb – Stock Options. Johnson & Johnson – Stock Options. Vibrant Advisory Board – Advisory Committee/Board Member.Judy Nee indicated no relevant financial relationships.
Vikram Rangan, MD1, Colin Wu, BS1, Johanna Iturrino, MD2, Anthony J. Lembo, MD3, Judy Nee, MD1. P4047 - Pilot Study of Crofelemer for Functional Diarrhea, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


