Tuesday Poster Session
Category: General Endoscopy
P4091 - Debunking Endoscopy Myths: GLP1-RA Use and GI Symptoms Do NOT Predict Bowel Preparation Success
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AW
Anshu Wadehra, MD
Detroit Medical Center/Wayne State University
Detroit, MI
Presenting Author(s)
Anshu Wadehra, MD1, Fadi Antaki, MD2, Ziad Kanaan, MD, PhD3, Samara Rifkin, MD1, Kirthi Lilley, MD3
1Detroit Medical Center/Wayne State University, Detroit, MI; 2John D. Dingell VA Medical Center and Wayne State University School of Medicine, Detroit, MI; 3John D. Dingell VA Medical Center, Detroit, MI
Introduction: Glucagon-like-peptide-1 receptor agonists (GLP1-RAs) are effective for managing type 2 diabetes and obesity by promoting satiety and delaying gastrointestinal motility. Concerns have arisen that GLP1-RAs may cause inadequate bowel preparation for colonoscopy. We aim to assess the rates of inadequate bowel preparation in patients on GLP1-RAs during colonoscopy and determine if peri-operative GI symptoms or GLP1-RA dose are additional risk factors.
Methods: We conducted a retrospective chart review at a single endoscopy unit. Patients who underwent colonoscopy between 2018 and 2023, on uninterrupted treatment with GLP1-RAs, were identified. Bowel preparation included a clear liquid diet for 24 hours and a split dose polyethylene glycol 3350 regimen. Patient data, including operative reports, were reviewed to assess demographics, GLP1-RA dose, peri-operative upper GI symptoms, and bowel preparation adequacy per the Boston Bowel Preparation Scale (BBPS). Adequate bowel preparation was defined as a BBPS score of ≥2 in every colon segment. These outcomes were compared to patients not on GLP1-RAs using Fisher's exact test.
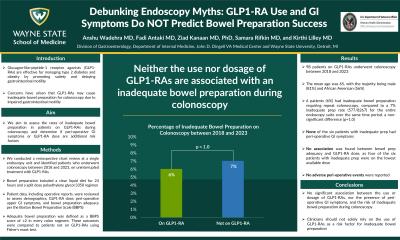
Results: Ninety-eight patients on GLP1-RAs underwent colonoscopy between 2018 and 2023. The mean age was 65, with the majority being male (81%) and African American (56%). Six patients (6%) had inadequate bowel preparation requiring repeat colonoscopy, compared to a 7% inadequate prep rate (577/8267) for the entire endoscopy suite over the same time period, a non-significant difference (p=1.0). None of the six patients with inadequate prep had peri-operative GI symptoms. However, three patients with adequate prep exhibited GI symptoms prior to endoscopy (post-prandial fullness, abdominal pain, and nausea). No association was found between bowel prep adequacy and GLP1-RA dose, as four of the six patients with inadequate prep were on the lowest available dose. No adverse peri-operative events were reported.
Discussion: Our findings indicate that neither the use or dosage of GLP1-RAs, nor the presence of peri-operative GI symptoms, are associated with an inadequate bowel preparation during colonoscopy. Therefore, clinicians should not solely rely on the use of GLP1-RAs as a risk factor for inadequate bowel preparation.
Disclosures:
Anshu Wadehra, MD1, Fadi Antaki, MD2, Ziad Kanaan, MD, PhD3, Samara Rifkin, MD1, Kirthi Lilley, MD3. P4091 - Debunking Endoscopy Myths: GLP1-RA Use and GI Symptoms Do NOT Predict Bowel Preparation Success, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Detroit Medical Center/Wayne State University, Detroit, MI; 2John D. Dingell VA Medical Center and Wayne State University School of Medicine, Detroit, MI; 3John D. Dingell VA Medical Center, Detroit, MI
Introduction: Glucagon-like-peptide-1 receptor agonists (GLP1-RAs) are effective for managing type 2 diabetes and obesity by promoting satiety and delaying gastrointestinal motility. Concerns have arisen that GLP1-RAs may cause inadequate bowel preparation for colonoscopy. We aim to assess the rates of inadequate bowel preparation in patients on GLP1-RAs during colonoscopy and determine if peri-operative GI symptoms or GLP1-RA dose are additional risk factors.
Methods: We conducted a retrospective chart review at a single endoscopy unit. Patients who underwent colonoscopy between 2018 and 2023, on uninterrupted treatment with GLP1-RAs, were identified. Bowel preparation included a clear liquid diet for 24 hours and a split dose polyethylene glycol 3350 regimen. Patient data, including operative reports, were reviewed to assess demographics, GLP1-RA dose, peri-operative upper GI symptoms, and bowel preparation adequacy per the Boston Bowel Preparation Scale (BBPS). Adequate bowel preparation was defined as a BBPS score of ≥2 in every colon segment. These outcomes were compared to patients not on GLP1-RAs using Fisher's exact test.
Results: Ninety-eight patients on GLP1-RAs underwent colonoscopy between 2018 and 2023. The mean age was 65, with the majority being male (81%) and African American (56%). Six patients (6%) had inadequate bowel preparation requiring repeat colonoscopy, compared to a 7% inadequate prep rate (577/8267) for the entire endoscopy suite over the same time period, a non-significant difference (p=1.0). None of the six patients with inadequate prep had peri-operative GI symptoms. However, three patients with adequate prep exhibited GI symptoms prior to endoscopy (post-prandial fullness, abdominal pain, and nausea). No association was found between bowel prep adequacy and GLP1-RA dose, as four of the six patients with inadequate prep were on the lowest available dose. No adverse peri-operative events were reported.
Discussion: Our findings indicate that neither the use or dosage of GLP1-RAs, nor the presence of peri-operative GI symptoms, are associated with an inadequate bowel preparation during colonoscopy. Therefore, clinicians should not solely rely on the use of GLP1-RAs as a risk factor for inadequate bowel preparation.
Disclosures:
Anshu Wadehra indicated no relevant financial relationships.
Fadi Antaki indicated no relevant financial relationships.
Ziad Kanaan indicated no relevant financial relationships.
Samara Rifkin indicated no relevant financial relationships.
Kirthi Lilley indicated no relevant financial relationships.
Anshu Wadehra, MD1, Fadi Antaki, MD2, Ziad Kanaan, MD, PhD3, Samara Rifkin, MD1, Kirthi Lilley, MD3. P4091 - Debunking Endoscopy Myths: GLP1-RA Use and GI Symptoms Do NOT Predict Bowel Preparation Success, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
