Tuesday Poster Session
Category: General Endoscopy
P4092 - Clear Liquid Diet and Colonoscopy Bowel Preparation Are Effective in Eliminating Retained Gastric Contents in Those on GLP1-RA Agents Undergoing Endoscopic Procedures
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AW
Anshu Wadehra, MD
Detroit Medical Center/Wayne State University
Detroit, MI
Presenting Author(s)
Anshu Wadehra, MD1, Fadi Antaki, MD2, Ziad Kanaan, MD, PhD3, Samara Rifkin, MD1, Kirthi Lilley, MD3
1Detroit Medical Center/Wayne State University, Detroit, MI; 2John D. Dingell VA Medical Center and Wayne State University School of Medicine, Detroit, MI; 3John D. Dingell VA Medical Center, Detroit, MI
Introduction: Glucagon-like-peptide-1 receptor agonists (GLP1-RAs) have gained significant popularity due to their use in type 2 diabetes and obesity management. Concerns for safety during endoscopic and surgical procedures have been raised due to their effect on gastric motility. This study aims to assess the rate of retained gastric contents (RGC) and peri-operative complications in those on uninterrupted treatment with GLP1-RAs undergoing upper endoscopy.
Methods: We conducted a retrospective chart review at a single endoscopy unit. Patients on GLP1-RAs who underwent EGD, with or without same-day colonoscopy, from 2018 to 2023 were identified. Patient charts and operative reports were manually reviewed to assess demographic information, dose of GLP1-RAs, presence of peri-operative upper GI symptoms, and presence of RGC on endoscopy.
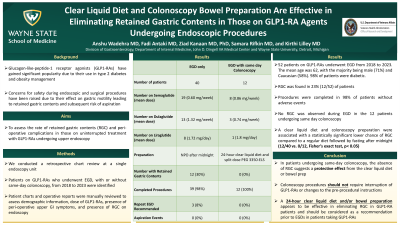
Results: Fifty-two patients on GLP1-RAs underwent EGD from 2018 to 2023. The mean age was 62, with the majority being male (71%) and Caucasian (58%). Ninety eight percent of patients were diabetic. RGC was found in 23% (12/52) of patients, with only one patient reporting pre-operative GI symptoms (abdominal pain and vomiting). There was no correlation between GLP1-RA dose and RGC. One EGD was aborted due to large RGC. No adverse events were reported.
Additionally, 12 out of the 52 patients underwent same-day colonoscopy with their EGD. These patients followed a clear liquid diet for 24 hours and received a split dose polyethylene glycol 3350-electrolyte solution for bowel preparation, completed 2 hours prior to procedure. No RGC was observed during EGD in these patients. Thus, a clear liquid diet and colonoscopy preparation were significantly associated with a lower chance of RGC compared to a regular diet followed by fasting after midnight (12/40 vs. 0/12, Fisher’s exact test, p< 0.05).
Discussion: Our findings indicate GLP1-RAs are associated with high rates of RGC during EGD. The clinical significance is unclear as most procedures could be completed, and no aspiration was documented. In patients undergoing same-day colonoscopy, the absence of RGC suggests a protective effect from the clear liquid diet or bowel preparation. As a result, colonoscopy procedures should not require interruption of GLP1-RAs or changes to the pre-procedural instructions. A 24-hour clear liquid diet appears to be effective in eliminating RGC in GLP1-RA patients and should be considered as a recommendation prior to EGDs in patients taking GLP1-RAs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Anshu Wadehra, MD1, Fadi Antaki, MD2, Ziad Kanaan, MD, PhD3, Samara Rifkin, MD1, Kirthi Lilley, MD3. P4092 - Clear Liquid Diet and Colonoscopy Bowel Preparation Are Effective in Eliminating Retained Gastric Contents in Those on GLP1-RA Agents Undergoing Endoscopic Procedures, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Detroit Medical Center/Wayne State University, Detroit, MI; 2John D. Dingell VA Medical Center and Wayne State University School of Medicine, Detroit, MI; 3John D. Dingell VA Medical Center, Detroit, MI
Introduction: Glucagon-like-peptide-1 receptor agonists (GLP1-RAs) have gained significant popularity due to their use in type 2 diabetes and obesity management. Concerns for safety during endoscopic and surgical procedures have been raised due to their effect on gastric motility. This study aims to assess the rate of retained gastric contents (RGC) and peri-operative complications in those on uninterrupted treatment with GLP1-RAs undergoing upper endoscopy.
Methods: We conducted a retrospective chart review at a single endoscopy unit. Patients on GLP1-RAs who underwent EGD, with or without same-day colonoscopy, from 2018 to 2023 were identified. Patient charts and operative reports were manually reviewed to assess demographic information, dose of GLP1-RAs, presence of peri-operative upper GI symptoms, and presence of RGC on endoscopy.
Results: Fifty-two patients on GLP1-RAs underwent EGD from 2018 to 2023. The mean age was 62, with the majority being male (71%) and Caucasian (58%). Ninety eight percent of patients were diabetic. RGC was found in 23% (12/52) of patients, with only one patient reporting pre-operative GI symptoms (abdominal pain and vomiting). There was no correlation between GLP1-RA dose and RGC. One EGD was aborted due to large RGC. No adverse events were reported.
Additionally, 12 out of the 52 patients underwent same-day colonoscopy with their EGD. These patients followed a clear liquid diet for 24 hours and received a split dose polyethylene glycol 3350-electrolyte solution for bowel preparation, completed 2 hours prior to procedure. No RGC was observed during EGD in these patients. Thus, a clear liquid diet and colonoscopy preparation were significantly associated with a lower chance of RGC compared to a regular diet followed by fasting after midnight (12/40 vs. 0/12, Fisher’s exact test, p< 0.05).
Discussion: Our findings indicate GLP1-RAs are associated with high rates of RGC during EGD. The clinical significance is unclear as most procedures could be completed, and no aspiration was documented. In patients undergoing same-day colonoscopy, the absence of RGC suggests a protective effect from the clear liquid diet or bowel preparation. As a result, colonoscopy procedures should not require interruption of GLP1-RAs or changes to the pre-procedural instructions. A 24-hour clear liquid diet appears to be effective in eliminating RGC in GLP1-RA patients and should be considered as a recommendation prior to EGDs in patients taking GLP1-RAs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Anshu Wadehra indicated no relevant financial relationships.
Fadi Antaki indicated no relevant financial relationships.
Ziad Kanaan indicated no relevant financial relationships.
Samara Rifkin indicated no relevant financial relationships.
Kirthi Lilley indicated no relevant financial relationships.
Anshu Wadehra, MD1, Fadi Antaki, MD2, Ziad Kanaan, MD, PhD3, Samara Rifkin, MD1, Kirthi Lilley, MD3. P4092 - Clear Liquid Diet and Colonoscopy Bowel Preparation Are Effective in Eliminating Retained Gastric Contents in Those on GLP1-RA Agents Undergoing Endoscopic Procedures, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
