Tuesday Poster Session
Category: GI Bleeding
P4175 - Characteristics of Upper Gastrointestinal Bleed Studies Registered in Clinicaltrials.gov – A Cross Sectional Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AT
Akanksha Togra, MD
Texas Tech University Health Sciences Center
El Paso, Texas
Presenting Author(s)
Akanksha Togra, MD, Swati Mahapatra, DO, Alejandro Robles, MD, Marc Zuckerman, MD, Sherif E.. Elhanafi, MD
Texas Tech University Health Sciences Center, El Paso, TX
Introduction: Upper GI bleeding (UGIB) rates are estimated at 40-150 cases per 100,000 individuals annually with mortality rates ranging from 5-10%. Thus, well designed clinical trials become imperative to improve patient outcomes in UGIB. The aim of this study was to analyse the trends of UGIB clinical studies registered at clinicaltrials.gov (CTG).
Methods: This is a cross-sectional study of all UGIB clinical studies registered in CTG from September 27, 2007 to April 30, 2024, identified using the ‘Advanced Search’ feature. Trial characteristics and trends were assessed through relative frequency calculations. Odds ratio and 95% confidence intervals were determined by comparing with overall studies.
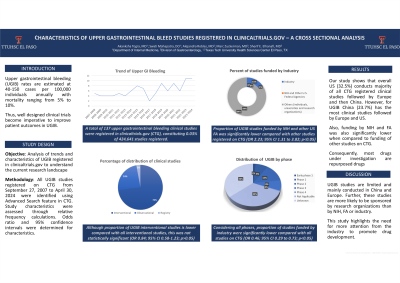
Results: A total of 137 UGIB clinical studies were registered in CTG, constituting 0.03% of 424,641 studies registered. Studies were conducted in various populations including peptic ulcer, variceal bleeding, infection and drug related injuries. The interventional studies were 100 (72.99%) while observational were 33 (27%), of which patient registries constituted 10.8%.
The proportion of UGIB interventional studies was lower compared with all other interventional studies (72.99 % vs 76.22%). However, this was not statistically significant (OR 0.84; 95 % CI: 0.58 to 1.23; P > 0.05).
US conducted majority of all CTG registered clinical studies (32.5%) followed by Europe (29.8%) and then China (14.7%). However in UGIB studies, China has the most clinical studies (23.7%) followed by Europe (19.1%) and US (16.0%).
A total of 21 (15.3%) UGIB studies were sponsored by industry; 2 (1.5%) by the NIH and other US Federal agencies (FA); and 122 (89.1%) by all others (individuals, universities, research organizations). The proportion of UGIB studies funded by industry was significantly lower, compared with all other studies registered on CTG (OR 0.46; 95 % CI: 0.29 to 0.73; P < 0.05). Funding trends by NIH and FA was also significantly lower (OR 0.24; 95 % CI: 0.06 to 0.98; P < 0.05). Majority of the studies are by others (OR 2.23; 95 % CI: 1.31 to 3.82; P < 0.05). Further, most of the drugs under investigation are repurposed drugs.
Discussion: UGIB related clinical studies registered in CTG are limited and mainly conducted in China and Europe. Further, these studies are more likely sponsored by universities, research organizations than by NIH, FA or industry. This study highlights the need for more attention from industry, NIH and FA to address UGIB, promote new drug development and improve clinical outcomes.
Disclosures:
Akanksha Togra, MD, Swati Mahapatra, DO, Alejandro Robles, MD, Marc Zuckerman, MD, Sherif E.. Elhanafi, MD. P4175 - Characteristics of Upper Gastrointestinal Bleed Studies Registered in Clinicaltrials.gov – A Cross Sectional Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Texas Tech University Health Sciences Center, El Paso, TX
Introduction: Upper GI bleeding (UGIB) rates are estimated at 40-150 cases per 100,000 individuals annually with mortality rates ranging from 5-10%. Thus, well designed clinical trials become imperative to improve patient outcomes in UGIB. The aim of this study was to analyse the trends of UGIB clinical studies registered at clinicaltrials.gov (CTG).
Methods: This is a cross-sectional study of all UGIB clinical studies registered in CTG from September 27, 2007 to April 30, 2024, identified using the ‘Advanced Search’ feature. Trial characteristics and trends were assessed through relative frequency calculations. Odds ratio and 95% confidence intervals were determined by comparing with overall studies.
Results: A total of 137 UGIB clinical studies were registered in CTG, constituting 0.03% of 424,641 studies registered. Studies were conducted in various populations including peptic ulcer, variceal bleeding, infection and drug related injuries. The interventional studies were 100 (72.99%) while observational were 33 (27%), of which patient registries constituted 10.8%.
The proportion of UGIB interventional studies was lower compared with all other interventional studies (72.99 % vs 76.22%). However, this was not statistically significant (OR 0.84; 95 % CI: 0.58 to 1.23; P > 0.05).
US conducted majority of all CTG registered clinical studies (32.5%) followed by Europe (29.8%) and then China (14.7%). However in UGIB studies, China has the most clinical studies (23.7%) followed by Europe (19.1%) and US (16.0%).
A total of 21 (15.3%) UGIB studies were sponsored by industry; 2 (1.5%) by the NIH and other US Federal agencies (FA); and 122 (89.1%) by all others (individuals, universities, research organizations). The proportion of UGIB studies funded by industry was significantly lower, compared with all other studies registered on CTG (OR 0.46; 95 % CI: 0.29 to 0.73; P < 0.05). Funding trends by NIH and FA was also significantly lower (OR 0.24; 95 % CI: 0.06 to 0.98; P < 0.05). Majority of the studies are by others (OR 2.23; 95 % CI: 1.31 to 3.82; P < 0.05). Further, most of the drugs under investigation are repurposed drugs.
Discussion: UGIB related clinical studies registered in CTG are limited and mainly conducted in China and Europe. Further, these studies are more likely sponsored by universities, research organizations than by NIH, FA or industry. This study highlights the need for more attention from industry, NIH and FA to address UGIB, promote new drug development and improve clinical outcomes.
Disclosures:
Akanksha Togra: Clinexel Inc – Owner/Ownership Interest. Clinexel Life Sciences Pvt Ltd – Owner/Ownership Interest. Cytenet Life science LLP – Owner/Ownership Interest. GLRK Healthcare foundation (Non-profit Organization Company) – Owner/Ownership Interest.
Swati Mahapatra indicated no relevant financial relationships.
Alejandro Robles indicated no relevant financial relationships.
Marc Zuckerman indicated no relevant financial relationships.
Sherif Elhanafi indicated no relevant financial relationships.
Akanksha Togra, MD, Swati Mahapatra, DO, Alejandro Robles, MD, Marc Zuckerman, MD, Sherif E.. Elhanafi, MD. P4175 - Characteristics of Upper Gastrointestinal Bleed Studies Registered in Clinicaltrials.gov – A Cross Sectional Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

