Tuesday Poster Session
Category: GI Bleeding
P4211 - Bound for Trouble: Acute Sevelamer-Induced GI Bleeding in a Non-CKD Patient
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- ER
Erik Roepke, MD
University of Alabama at Birmingham
Birmingham, AL
Presenting Author(s)
Erik Roepke, MD1, Rajesh Konjeti, MD2, Marijeta Pekez, MD2
1University of Alabama at Birmingham, Birmingham, AL; 2University of Kentucky, Lexington, KY
Introduction: Sevelamer, a phosphate binder often used to treat hyperphosphatemia, has rare known adverse effects that can include gastrointestinal (GI) ulceration. This is a case of sevelamer-associated cecal ulceration with hemorrhage in a patient with short-term sevelamer use and without preexisting CKD.
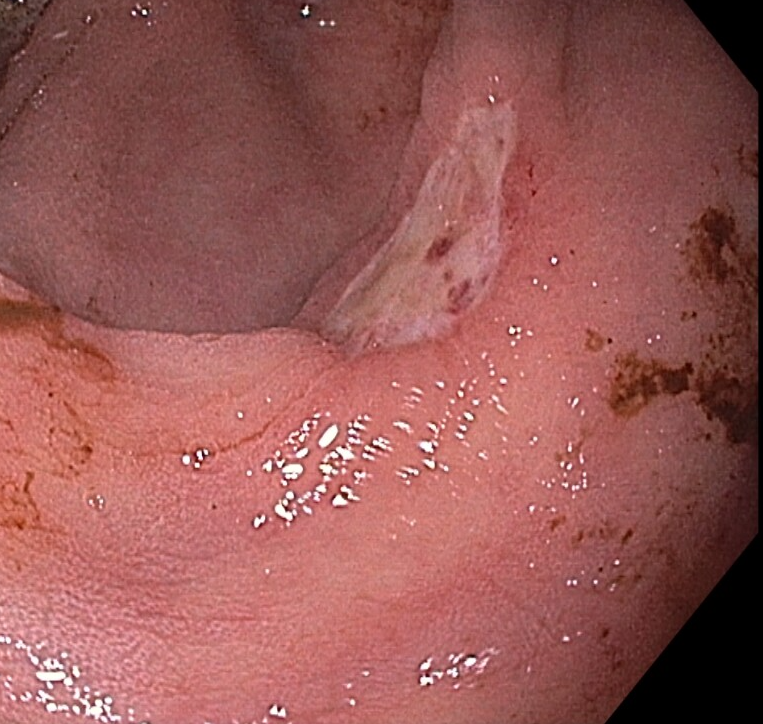
Case Description/Methods: A 55-year-old woman with HTN, HLD, COPD, and poorly controlled type II DM (last A1c was 16) was transferred to our institution with respiratory failure secondary to Influenza A and Streptococcus pneumoniae. Upon arrival, labs were significant for creatinine of 5.07 and BUN of 89. Two days later, patient was started on sevelamer carbonate 1,600 mg TID with meals due to phosphorus values up to 7.5 mg/dL. That night, the patient experienced multiple episodes of melena with hemoglobin dropping from 11.4 to 8.9. Vital signs remained stable. A non-contrast CT abdomen was unremarkable, and physical exam was limited due to the patient being intubated and sedated. Sigmoidoscopy showed a significant amount of old blood and a 15mm cratered, linear ulcer without active hemorrhage in the cecum. Biopsy results from the ulcer showed one crystalloid structure with tree bark appearance and rusty color, consistent with sevelamer. Sevelamer was promptly discontinued, and she was later discharged without further GI bleeding.
Discussion: We presented a case of cecal ulceration with hemorrhage, which we suspect was associated with usage of sevelamer. Other case reports that describe sevelamer-associated GI bleeding mostly include patients with a history of CKD, use of hemodialysis (HD), and/or long-term use of sevelamer. In contrast, our patient presented with an intrarenal AKI, did not require HD, and used sevelamer for less than one day prior to GI bleed. Further, this patient was started on the maximum dose of sevelamer due to phosphorus of 7.5 mg/dL, which is consistent with guidelines. However, it may have been advantageous to start at a lower dose given the severity of her illness and her borderline phosphorus value. We wonder if the adverse effects of sevelamer may be magnified, and the risk of ischemia heightened, in fragile hospitalized patients. Caution should be exercised when deciding the dose of sevelamer in an acutely ill patient, and prompt cessation of the drug should be considered in patients taking sevelamer who present with similar symptoms.
Disclosures:
Erik Roepke, MD1, Rajesh Konjeti, MD2, Marijeta Pekez, MD2. P4211 - Bound for Trouble: Acute Sevelamer-Induced GI Bleeding in a Non-CKD Patient, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Alabama at Birmingham, Birmingham, AL; 2University of Kentucky, Lexington, KY
Introduction: Sevelamer, a phosphate binder often used to treat hyperphosphatemia, has rare known adverse effects that can include gastrointestinal (GI) ulceration. This is a case of sevelamer-associated cecal ulceration with hemorrhage in a patient with short-term sevelamer use and without preexisting CKD.
Case Description/Methods: A 55-year-old woman with HTN, HLD, COPD, and poorly controlled type II DM (last A1c was 16) was transferred to our institution with respiratory failure secondary to Influenza A and Streptococcus pneumoniae. Upon arrival, labs were significant for creatinine of 5.07 and BUN of 89. Two days later, patient was started on sevelamer carbonate 1,600 mg TID with meals due to phosphorus values up to 7.5 mg/dL. That night, the patient experienced multiple episodes of melena with hemoglobin dropping from 11.4 to 8.9. Vital signs remained stable. A non-contrast CT abdomen was unremarkable, and physical exam was limited due to the patient being intubated and sedated. Sigmoidoscopy showed a significant amount of old blood and a 15mm cratered, linear ulcer without active hemorrhage in the cecum. Biopsy results from the ulcer showed one crystalloid structure with tree bark appearance and rusty color, consistent with sevelamer. Sevelamer was promptly discontinued, and she was later discharged without further GI bleeding.
Discussion: We presented a case of cecal ulceration with hemorrhage, which we suspect was associated with usage of sevelamer. Other case reports that describe sevelamer-associated GI bleeding mostly include patients with a history of CKD, use of hemodialysis (HD), and/or long-term use of sevelamer. In contrast, our patient presented with an intrarenal AKI, did not require HD, and used sevelamer for less than one day prior to GI bleed. Further, this patient was started on the maximum dose of sevelamer due to phosphorus of 7.5 mg/dL, which is consistent with guidelines. However, it may have been advantageous to start at a lower dose given the severity of her illness and her borderline phosphorus value. We wonder if the adverse effects of sevelamer may be magnified, and the risk of ischemia heightened, in fragile hospitalized patients. Caution should be exercised when deciding the dose of sevelamer in an acutely ill patient, and prompt cessation of the drug should be considered in patients taking sevelamer who present with similar symptoms.

Figure: Cecal ulcer
Disclosures:
Erik Roepke indicated no relevant financial relationships.
Rajesh Konjeti indicated no relevant financial relationships.
Marijeta Pekez indicated no relevant financial relationships.
Erik Roepke, MD1, Rajesh Konjeti, MD2, Marijeta Pekez, MD2. P4211 - Bound for Trouble: Acute Sevelamer-Induced GI Bleeding in a Non-CKD Patient, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
