Tuesday Poster Session
Category: Functional Bowel Disease
P4059 - Fodzyme at Its Finest: A Case Series Exploring a New Option for IBS Patients
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Alexander Kaye, MD, MBA
SUNY Downstate Health Sciences University
Brooklyn, NY
Presenting Author(s)
Alexander Kaye, MD, MBA1, Sarah Meyers, DO2, David Hachuel, MPH3, Jocelyn Wells, MS, RDN3, Thomas Wallach, MD1, Savanna Thor, DO, MPH1
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2Robert Wood Johnson Medical School, Rutgers University, Piscataway, NJ; 3Kiwi Biosciences, Cambridge, MA
Introduction: Disorders of the gut-brain interaction (DGBI) have a significant impact on patients’ mental and physical wellness. One of the most common types of DGBI is irritable bowel syndrome (IBS). A common approach to treating IBS is a diet low in fermentable galacto-oligosaccharides (GOS), disaccharides, monosaccharides, and polyols (FODMAPs) which helps avoid common food triggers of symptoms, however it can be challenging to adhere to. A new supplement, FODZYME, contains a digestive enzyme blend that breaks down the fructan, GOS, and lactose FODMAP groups and may allow for co-ingestion of FODMAPs while controlling symptoms. While FODZYME has been studied in-vitro, it has not been explored in a human population until this study.
Methods: This study is a real world self-controlled case series to assess the efficacy of FODZYME in treating IBS. This study was exempt from IRB approval. Participants included patients who trialed FODZYME and completed an initial survey regarding IBS symptoms including questions about IBS-subjective global assessment (IBS-SGA); the IBS-visual analogue scales (IBS-VAS) for bloating, disruption to daily life and mental wellbeing; and the IBS Quality of Life (IBS-QoL) Food Avoidance sub-scale. After 4 weeks, patients completed an identical follow-up survey. Paired t-tests were used to compare mean differences and means respectively. A Bonferroni correction was applied to account for the number of outcomes examined.
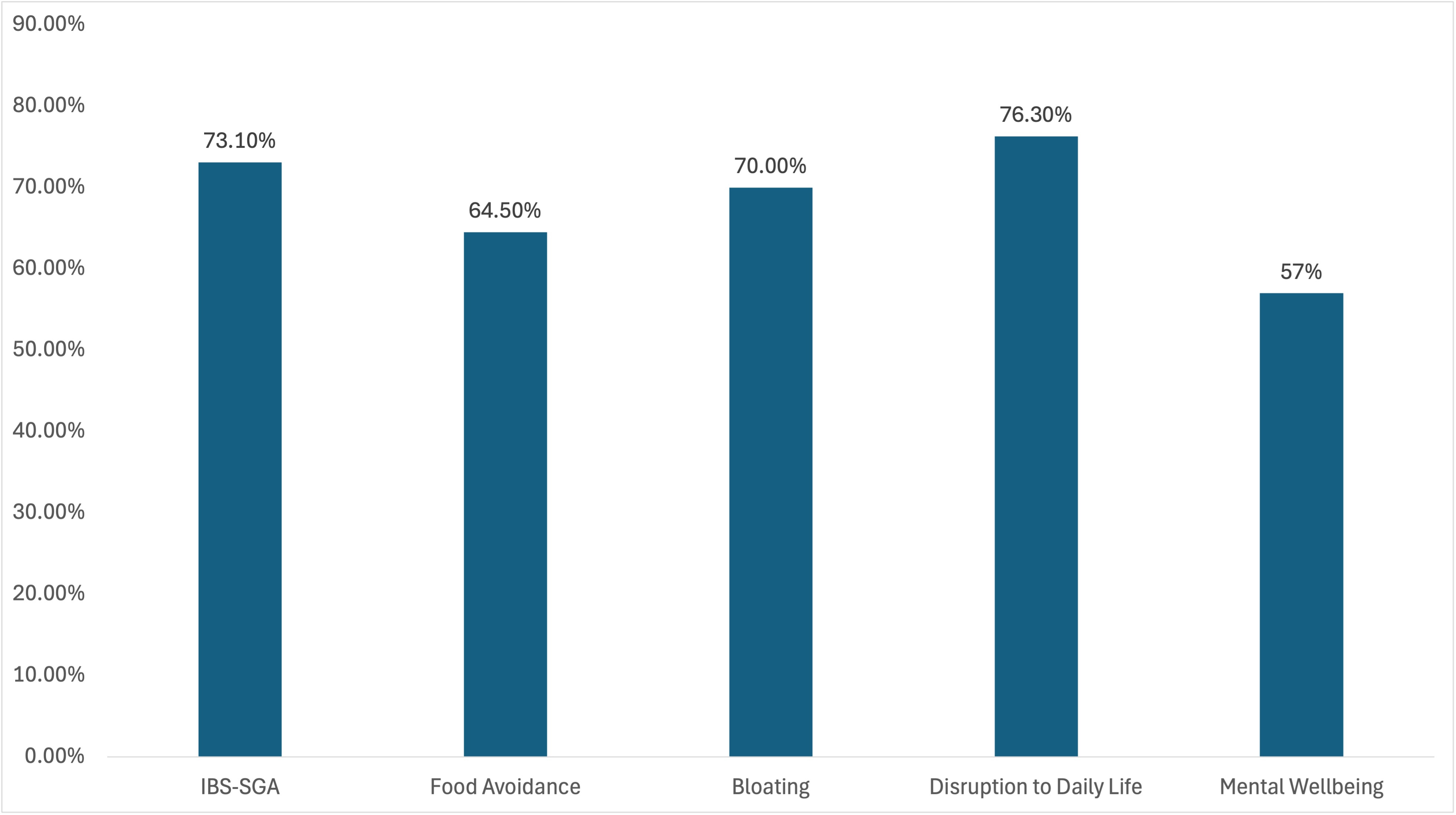
Results: Out of 527 patients beginning the trial, 131 completed the study. Among those who completed the study, 93 reported a history of IBS. Of these, the mean age was 56.6 years old, 83.9% were women, and 100% reported a history of IBS. After 4-weeks of using FODZYME at least once, there was clinically-significant improvement in the IBS-SGA (73.1% experienced improvement, with a mean improvement of 25.7%, p-value < 0.001), bloating (70.0% experienced decreased frequency with a mean decrease of 28.8%, p-value < 0.001), disruption to daily life (76.3% experienced fewer daily disruptions with a mean decrease of 27.8%, p< 0.001), food avoidance (64.5% experienced improvement with a mean improvement of 21.6%, p< 0.001), and mental wellbeing (57.0% reporting improvement with a mean rating scale improvement of 14.3%, p-value< 0.001).
Discussion: FODZYME demonstrated efficacy in improving the quality of life, mental health and physical wellness in a patient population with a high prevalence of IBS. Further studies can better establish the efficacy of FODZYME.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Alexander Kaye, MD, MBA1, Sarah Meyers, DO2, David Hachuel, MPH3, Jocelyn Wells, MS, RDN3, Thomas Wallach, MD1, Savanna Thor, DO, MPH1. P4059 - Fodzyme at Its Finest: A Case Series Exploring a New Option for IBS Patients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2Robert Wood Johnson Medical School, Rutgers University, Piscataway, NJ; 3Kiwi Biosciences, Cambridge, MA
Introduction: Disorders of the gut-brain interaction (DGBI) have a significant impact on patients’ mental and physical wellness. One of the most common types of DGBI is irritable bowel syndrome (IBS). A common approach to treating IBS is a diet low in fermentable galacto-oligosaccharides (GOS), disaccharides, monosaccharides, and polyols (FODMAPs) which helps avoid common food triggers of symptoms, however it can be challenging to adhere to. A new supplement, FODZYME, contains a digestive enzyme blend that breaks down the fructan, GOS, and lactose FODMAP groups and may allow for co-ingestion of FODMAPs while controlling symptoms. While FODZYME has been studied in-vitro, it has not been explored in a human population until this study.
Methods: This study is a real world self-controlled case series to assess the efficacy of FODZYME in treating IBS. This study was exempt from IRB approval. Participants included patients who trialed FODZYME and completed an initial survey regarding IBS symptoms including questions about IBS-subjective global assessment (IBS-SGA); the IBS-visual analogue scales (IBS-VAS) for bloating, disruption to daily life and mental wellbeing; and the IBS Quality of Life (IBS-QoL) Food Avoidance sub-scale. After 4 weeks, patients completed an identical follow-up survey. Paired t-tests were used to compare mean differences and means respectively. A Bonferroni correction was applied to account for the number of outcomes examined.
Results: Out of 527 patients beginning the trial, 131 completed the study. Among those who completed the study, 93 reported a history of IBS. Of these, the mean age was 56.6 years old, 83.9% were women, and 100% reported a history of IBS. After 4-weeks of using FODZYME at least once, there was clinically-significant improvement in the IBS-SGA (73.1% experienced improvement, with a mean improvement of 25.7%, p-value < 0.001), bloating (70.0% experienced decreased frequency with a mean decrease of 28.8%, p-value < 0.001), disruption to daily life (76.3% experienced fewer daily disruptions with a mean decrease of 27.8%, p< 0.001), food avoidance (64.5% experienced improvement with a mean improvement of 21.6%, p< 0.001), and mental wellbeing (57.0% reporting improvement with a mean rating scale improvement of 14.3%, p-value< 0.001).
Discussion: FODZYME demonstrated efficacy in improving the quality of life, mental health and physical wellness in a patient population with a high prevalence of IBS. Further studies can better establish the efficacy of FODZYME.

Figure: Percent of Patients Reporting Improvement with FODZYME use
*IBS-subjective global assessment (IBS-SGA)
*IBS-subjective global assessment (IBS-SGA)
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Alexander Kaye indicated no relevant financial relationships.
Sarah Meyers indicated no relevant financial relationships.
David Hachuel: Kiwi Biosciences – Owner/Ownership Interest, Stock-privately held company.
Jocelyn Wells: Kiwi Biosciences – Employee, Stock Options.
Thomas Wallach: Kiwi Bioscience – Advisor or Review Panel Member, Owner/Ownership Interest. Nutricia Danone – Speakers Bureau. Regeneron – Speakers Bureau.
Savanna Thor indicated no relevant financial relationships.
Alexander Kaye, MD, MBA1, Sarah Meyers, DO2, David Hachuel, MPH3, Jocelyn Wells, MS, RDN3, Thomas Wallach, MD1, Savanna Thor, DO, MPH1. P4059 - Fodzyme at Its Finest: A Case Series Exploring a New Option for IBS Patients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

