Tuesday Poster Session
Category: Functional Bowel Disease
P4062 - Demographic Disparities of Clinical Trials Focused on Functional Gastrointestinal Disorders
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Khushboo Gala, MBBS
Fellow
Mayo Clinic School of Graduate Medical Education
Rochester, MN
Presenting Author(s)
Khushboo Gala, MBBS1, Hector F.. Reyes Santiago, 2, Wissam Ghusn, MD3, Raseen Tariq, MBBS4, Jean Fox, MD4, Victor Chedid, MD, MS4
1Mayo Clinic School of Graduate Medical Education, Rochester, MN; 2University of Puerto Rico School of Medicine, Guaynabo, Puerto Rico; 3Boston Medical Center, Boston, MA; 4Mayo Clinic, Rochester, MN
Introduction: Functional gastrointestinal disorders (FGIDs) affect about 40% of the United States and global population, affecting patients of every age, sex, and racial and ethnic background. There is a paucity of data examining the populations recruited in clinical trials focused on FGID. Disparities in recruitment for clinical trials across medical specialties have been noted, which makes it challenging to translate their findings to the real world. We performed a systematic review of trials focused on FGIDs and examined the sex, racial and ethnic background, and age of participants.
Methods: We performed a systematic search of trials focused on FGIDs on ClinicalTrials.Gov, from inception to June 1, 2023. Specifically, we included trials focused on constipation, diarrhea, abdominal pain, irritable bowel syndrome, and esophageal dysmotility. Incomplete trials, trials conducted outside the United States and in languages other than English were excluded. Demographic details from all trials were abstracted and recorded. Descriptive analysis was performed with continuous data summarized as the mean and standard deviation (SD) and categorical data as percentages and values.
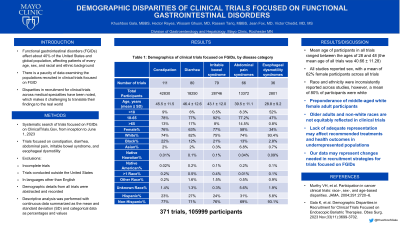
Results: We included a total of 371 trials, with a total of 105999 participants, with the maximum number of participants in trials related to irritable bowel syndrome and constipation (Table 1). The mean age of participants in all trials ranged between the ages of 28 and 48 (the mean age of all trials was 40.66 ± 11.28). All studies reported sex, with a mean of 62% female participants across all trials. Race and ethnicity were inconsistently reported across studies, however, a mean of 80% of participants were white.
Discussion: We found a preponderance of middle-aged white female adult participants in trials focused on FGIDs. Although it is difficult to draw exact comparisons of this against the prevalence of FGIDs in the community given the lack of high-quality data regarding the same, our data noted multiple disparities in these participants. Specifically, older adults and non-white races are not equitably reflected in clinical trials. Lack of adequate representation may affect recommended treatments and health outcomes in underrepresented populations; our data may represent changes needed in recruitment strategies for trials focused on FGIDs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Khushboo Gala, MBBS1, Hector F.. Reyes Santiago, 2, Wissam Ghusn, MD3, Raseen Tariq, MBBS4, Jean Fox, MD4, Victor Chedid, MD, MS4. P4062 - Demographic Disparities of Clinical Trials Focused on Functional Gastrointestinal Disorders, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mayo Clinic School of Graduate Medical Education, Rochester, MN; 2University of Puerto Rico School of Medicine, Guaynabo, Puerto Rico; 3Boston Medical Center, Boston, MA; 4Mayo Clinic, Rochester, MN
Introduction: Functional gastrointestinal disorders (FGIDs) affect about 40% of the United States and global population, affecting patients of every age, sex, and racial and ethnic background. There is a paucity of data examining the populations recruited in clinical trials focused on FGID. Disparities in recruitment for clinical trials across medical specialties have been noted, which makes it challenging to translate their findings to the real world. We performed a systematic review of trials focused on FGIDs and examined the sex, racial and ethnic background, and age of participants.
Methods: We performed a systematic search of trials focused on FGIDs on ClinicalTrials.Gov, from inception to June 1, 2023. Specifically, we included trials focused on constipation, diarrhea, abdominal pain, irritable bowel syndrome, and esophageal dysmotility. Incomplete trials, trials conducted outside the United States and in languages other than English were excluded. Demographic details from all trials were abstracted and recorded. Descriptive analysis was performed with continuous data summarized as the mean and standard deviation (SD) and categorical data as percentages and values.
Results: We included a total of 371 trials, with a total of 105999 participants, with the maximum number of participants in trials related to irritable bowel syndrome and constipation (Table 1). The mean age of participants in all trials ranged between the ages of 28 and 48 (the mean age of all trials was 40.66 ± 11.28). All studies reported sex, with a mean of 62% female participants across all trials. Race and ethnicity were inconsistently reported across studies, however, a mean of 80% of participants were white.
Discussion: We found a preponderance of middle-aged white female adult participants in trials focused on FGIDs. Although it is difficult to draw exact comparisons of this against the prevalence of FGIDs in the community given the lack of high-quality data regarding the same, our data noted multiple disparities in these participants. Specifically, older adults and non-white races are not equitably reflected in clinical trials. Lack of adequate representation may affect recommended treatments and health outcomes in underrepresented populations; our data may represent changes needed in recruitment strategies for trials focused on FGIDs.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Khushboo Gala indicated no relevant financial relationships.
Hector Reyes Santiago indicated no relevant financial relationships.
Wissam Ghusn indicated no relevant financial relationships.
Raseen Tariq indicated no relevant financial relationships.
Jean Fox indicated no relevant financial relationships.
Victor Chedid indicated no relevant financial relationships.
Khushboo Gala, MBBS1, Hector F.. Reyes Santiago, 2, Wissam Ghusn, MD3, Raseen Tariq, MBBS4, Jean Fox, MD4, Victor Chedid, MD, MS4. P4062 - Demographic Disparities of Clinical Trials Focused on Functional Gastrointestinal Disorders, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
