Monday Poster Session
Category: Liver
P2905 - Diagnostic Accuracy of HCV Core Antigen Testing in Hemodialysis Patients: A Systematic Review and Meta-Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Shravya Ginnaram, MD
Jefferson Abington Hospital
Willow Grove, PA
Presenting Author(s)
Shravya Ginnaram, MD1, Dawood Tahir, MD1, Sudeep Nugooru, MD, MBA1, Pradeep Yarra, MD2, Shilpitha Janga, MBBS3, Stephanie Tzarnas, MD4, Maria Lagarde Mussa, MD4
1Jefferson Abington Hospital, Willow Grove, PA; 2Saint Louis University School of Medicine, St. Louis, MO; 3Siddhartha Medical College, Willow Grove, PA; 4Einstein Medical Center, Philadelphia, PA
Introduction: Hepatitis C virus (HCV) infection poses a significant global health challenge, particularly in hemodialysis (HD) patients where the need for frequent HCV RNA testing increases costs significantly, tripling them compared to direct HCV core antigen (HCVcAg) testing. Additionally, the effectiveness of anti-HCV antibody screening is suboptimal for HD and renal transplant patients due to their immunosuppressed state. The aim of this study is to evaluate the diagnostic performance of HCVcAg in detecting HCV infection in HD patients.
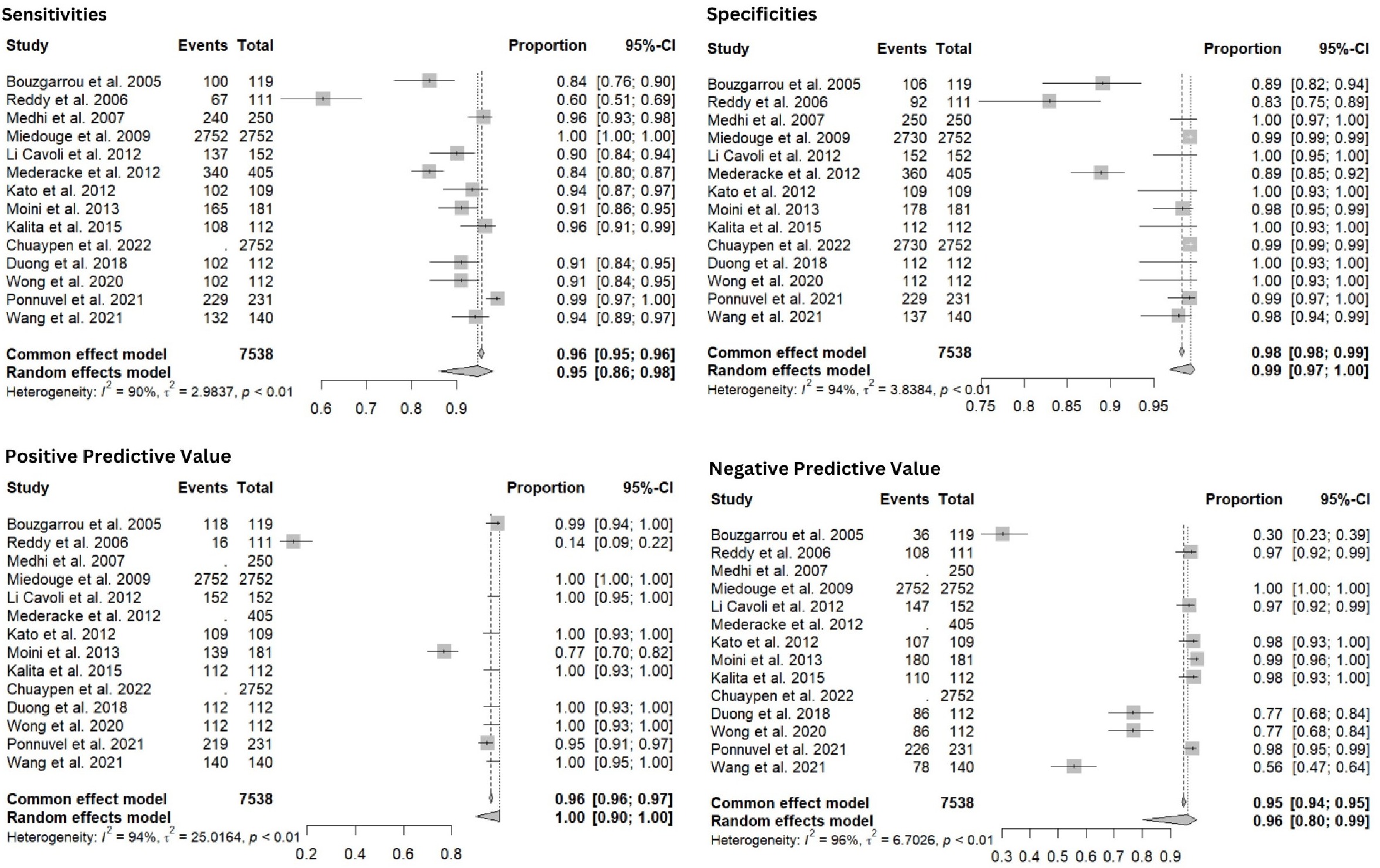
Methods: We conducted a comprehensive literature search using Cochrane library and MEDLINE/PubMed databases to identify studies that assessed the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of HCVcAg in HD patients. 14 studies, published between 2005 and 2022, met the inclusion criteria, with a combined sample size of 7,538 patients. Data was extracted and analyzed using R/R Studio [v4.4]. Meta-analyses were performed using a random-effects model, and heterogeneity was assessed using the I² statistic. Subgroup analyses were conducted based on publication year.
Results: The pooled sensitivity of HCVcAg was 95% (95% CI: 86%-98%) with significant heterogeneity (I² = 90%). The pooled specificity was 98% (95% CI: 97%-99%) with substantial heterogeneity (I² = 94%). Pooled PPV was 96% (95% CI: 80%-99%), and NPV was 95% (95% CI: 94%-95%), both showing high heterogeneity (I² = 96% and I² = 94%).
Subgroup analysis based on publication year showed: Pooled sensitivity of studies from 2005 to 2012 of 95% (95% CI: 86%-98%) with significant heterogeneity—I² = 92%. For studies published between 2013 and 2022, the combined sensitivity was reported as 95% (95% CI: 94%-98%) with substantial heterogeneity; I² = 64%.
Discussion: HCV RNA testing, using PCR-based target amplification, offers superior sensitivity, making it the gold standard. Whereas HCVcAg testing, with its high sensitivity and specificity, particularly in HD patients, is a valuable tool to confirm viral replication activity. Given its advantages in cost, fast analytical process, and turnaround time, HCVcAg testing could streamline screening and confirmation processes, especially in resource-scarce settings. Alternatively, combining HCV antibody screening with sequential HCVcAg and RNA testing can be implemented. Caution is warranted due to observed heterogeneity and potential publication bias, emphasizing the need for further validation in diverse clinical settings.
Disclosures:
Shravya Ginnaram, MD1, Dawood Tahir, MD1, Sudeep Nugooru, MD, MBA1, Pradeep Yarra, MD2, Shilpitha Janga, MBBS3, Stephanie Tzarnas, MD4, Maria Lagarde Mussa, MD4. P2905 - Diagnostic Accuracy of HCV Core Antigen Testing in Hemodialysis Patients: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Jefferson Abington Hospital, Willow Grove, PA; 2Saint Louis University School of Medicine, St. Louis, MO; 3Siddhartha Medical College, Willow Grove, PA; 4Einstein Medical Center, Philadelphia, PA
Introduction: Hepatitis C virus (HCV) infection poses a significant global health challenge, particularly in hemodialysis (HD) patients where the need for frequent HCV RNA testing increases costs significantly, tripling them compared to direct HCV core antigen (HCVcAg) testing. Additionally, the effectiveness of anti-HCV antibody screening is suboptimal for HD and renal transplant patients due to their immunosuppressed state. The aim of this study is to evaluate the diagnostic performance of HCVcAg in detecting HCV infection in HD patients.
Methods: We conducted a comprehensive literature search using Cochrane library and MEDLINE/PubMed databases to identify studies that assessed the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of HCVcAg in HD patients. 14 studies, published between 2005 and 2022, met the inclusion criteria, with a combined sample size of 7,538 patients. Data was extracted and analyzed using R/R Studio [v4.4]. Meta-analyses were performed using a random-effects model, and heterogeneity was assessed using the I² statistic. Subgroup analyses were conducted based on publication year.
Results: The pooled sensitivity of HCVcAg was 95% (95% CI: 86%-98%) with significant heterogeneity (I² = 90%). The pooled specificity was 98% (95% CI: 97%-99%) with substantial heterogeneity (I² = 94%). Pooled PPV was 96% (95% CI: 80%-99%), and NPV was 95% (95% CI: 94%-95%), both showing high heterogeneity (I² = 96% and I² = 94%).
Subgroup analysis based on publication year showed: Pooled sensitivity of studies from 2005 to 2012 of 95% (95% CI: 86%-98%) with significant heterogeneity—I² = 92%. For studies published between 2013 and 2022, the combined sensitivity was reported as 95% (95% CI: 94%-98%) with substantial heterogeneity; I² = 64%.
Discussion: HCV RNA testing, using PCR-based target amplification, offers superior sensitivity, making it the gold standard. Whereas HCVcAg testing, with its high sensitivity and specificity, particularly in HD patients, is a valuable tool to confirm viral replication activity. Given its advantages in cost, fast analytical process, and turnaround time, HCVcAg testing could streamline screening and confirmation processes, especially in resource-scarce settings. Alternatively, combining HCV antibody screening with sequential HCVcAg and RNA testing can be implemented. Caution is warranted due to observed heterogeneity and potential publication bias, emphasizing the need for further validation in diverse clinical settings.

Figure: Forest Plots Depicting Sensitivities, Specificities, Positive Predictive Value and Negative Predictive Value
Disclosures:
Shravya Ginnaram indicated no relevant financial relationships.
Dawood Tahir indicated no relevant financial relationships.
Sudeep Nugooru indicated no relevant financial relationships.
Pradeep Yarra indicated no relevant financial relationships.
Shilpitha Janga indicated no relevant financial relationships.
Stephanie Tzarnas indicated no relevant financial relationships.
Maria Lagarde Mussa indicated no relevant financial relationships.
Shravya Ginnaram, MD1, Dawood Tahir, MD1, Sudeep Nugooru, MD, MBA1, Pradeep Yarra, MD2, Shilpitha Janga, MBBS3, Stephanie Tzarnas, MD4, Maria Lagarde Mussa, MD4. P2905 - Diagnostic Accuracy of HCV Core Antigen Testing in Hemodialysis Patients: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
