Monday Poster Session
Category: Liver
P2935 - Efficacy and Safety of Ileal Bile Acid Transport Inhibitors in Inherited Cholestatic Liver Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- ME
Mohamed Elnaggar, MD
Hartford HealthCare
Hartford, CT
Presenting Author(s)
Muhammad Imran, MBBS1, Ahmed A.. Ibrahim, MBBch2, Mohamed Elnaggar, MD3, Shujaat Ali, MBBS1, Areeba Mehmood, MBBS4, Saba Khalil, MBBS5, Hadia Khan, MBBS1, Ahmed Bostamy Elsnhory, MBBCh6, Omar Saab, MD7, Mohamed Abuelazm, MD8
1University College of Medicine and Dentistry, The University of Lahore, Lahore, Punjab, Pakistan; 2Menoufia University, Menoufia, Al Minufiyah, Egypt; 3Hartford HealthCare, Hartford, CT; 4Sargodha Medical College, Sargodha, Punjab, Pakistan; 5Fatima Jinnah Medical University, Lahore, Punjab, Pakistan; 6Al-Azhar University, Cairo, Ad Daqahliyah, Egypt; 7Cleveland Clinic Foundation, Westlake, OH; 8Tanta University, Tanta, Al Gharbiyah, Egypt
Introduction: Inherited cholestatic liver disorders such as progressive familial intrahepatic cholestasis (PFIC) and Alagille syndrome result in significant pruritis and increased serum bile acids, necessitating liver transplantation. This study aims to evaluate the efficacy and safety of Ileal bile acid transport inhibitors (IBATIs) in children with PFIC and Alagille syndrome.
Methods: We conducted a comprehensive search across “Web of Science, SCOPUS, PubMed, and CENTRAL” from inception until May 30, 2023, to identify relevant randomized controlled trials (RCTs). The search results were imported into Covidence for article eligibility screening, and all relevant outcomes data were synthesized using risk ratios (RR) or mean differences (MD) with 95% confidence intervals (CIs) in meta-analysis models within RevMan 5.4.
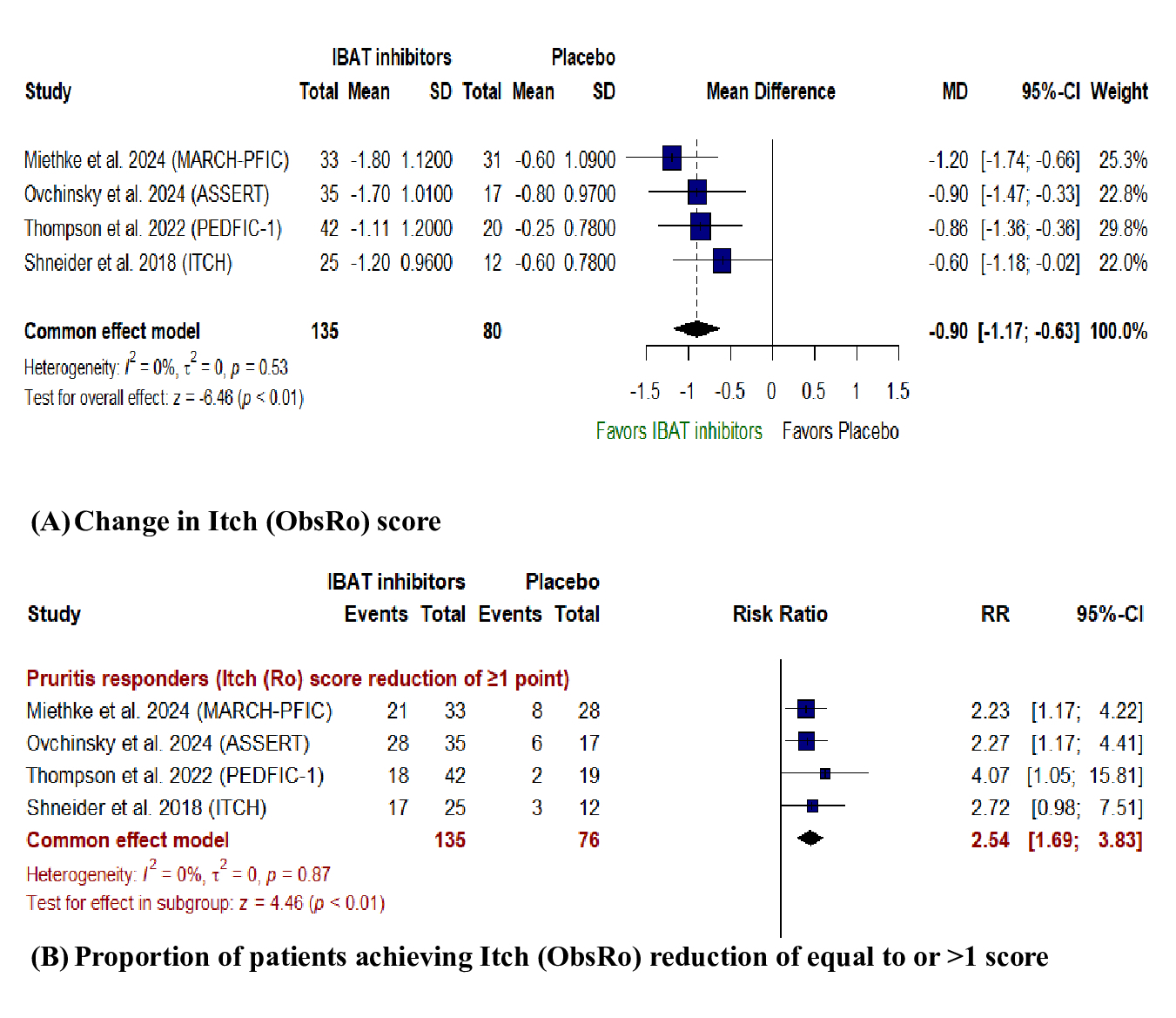
Results: Four multicentric RCTs involving 215 patients with PFIC and Alagille syndrome were included. IBATIs were associated with a significant reduction in itch observer reported outcome (Itch (ObsRo)) score (MD: - 0.90, 95% CI [-1.17, -0.63], P< 0.01) and a greater proportion of patients achieving ≥ 1 score reduction in Itch (ObsRo) score (RR: 2.54, 95% CI [3.83, 1.69], P< 0.01) compared to placebo. Also, significant reduction in serum bile acids (MD: -119.06, 95% CI [-152.37, -85.74], P< 0.01), total bilirubin (MD: - 0.73, 95% CI [-1.32, -0.15], P= 0.01) and serum cholesterol (MD: -1.97, 95% CI [-3.64, -0.31], P= 0.02) were observed with IBATIs. No difference was observed in any treatment-emergent adverse events (TEAs) (RR: 1.02, 95% CI [1.12, 0.93], P= 0.71), TEAs leading to drug discontinuation (1.03, 95% CI [5.56, 0.19], P= 0.97], any serious TEAs, and liver-related TEAs.
Discussion: IBATIs reduced the Itch (ObsRo) scores, serum bile acids, total bilirubin, and cholesterol with tolerable safety profiles, indicating their promising potential in managing inherited cholestatic liver disorders.
Disclosures:
Muhammad Imran, MBBS1, Ahmed A.. Ibrahim, MBBch2, Mohamed Elnaggar, MD3, Shujaat Ali, MBBS1, Areeba Mehmood, MBBS4, Saba Khalil, MBBS5, Hadia Khan, MBBS1, Ahmed Bostamy Elsnhory, MBBCh6, Omar Saab, MD7, Mohamed Abuelazm, MD8. P2935 - Efficacy and Safety of Ileal Bile Acid Transport Inhibitors in Inherited Cholestatic Liver Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University College of Medicine and Dentistry, The University of Lahore, Lahore, Punjab, Pakistan; 2Menoufia University, Menoufia, Al Minufiyah, Egypt; 3Hartford HealthCare, Hartford, CT; 4Sargodha Medical College, Sargodha, Punjab, Pakistan; 5Fatima Jinnah Medical University, Lahore, Punjab, Pakistan; 6Al-Azhar University, Cairo, Ad Daqahliyah, Egypt; 7Cleveland Clinic Foundation, Westlake, OH; 8Tanta University, Tanta, Al Gharbiyah, Egypt
Introduction: Inherited cholestatic liver disorders such as progressive familial intrahepatic cholestasis (PFIC) and Alagille syndrome result in significant pruritis and increased serum bile acids, necessitating liver transplantation. This study aims to evaluate the efficacy and safety of Ileal bile acid transport inhibitors (IBATIs) in children with PFIC and Alagille syndrome.
Methods: We conducted a comprehensive search across “Web of Science, SCOPUS, PubMed, and CENTRAL” from inception until May 30, 2023, to identify relevant randomized controlled trials (RCTs). The search results were imported into Covidence for article eligibility screening, and all relevant outcomes data were synthesized using risk ratios (RR) or mean differences (MD) with 95% confidence intervals (CIs) in meta-analysis models within RevMan 5.4.
Results: Four multicentric RCTs involving 215 patients with PFIC and Alagille syndrome were included. IBATIs were associated with a significant reduction in itch observer reported outcome (Itch (ObsRo)) score (MD: - 0.90, 95% CI [-1.17, -0.63], P< 0.01) and a greater proportion of patients achieving ≥ 1 score reduction in Itch (ObsRo) score (RR: 2.54, 95% CI [3.83, 1.69], P< 0.01) compared to placebo. Also, significant reduction in serum bile acids (MD: -119.06, 95% CI [-152.37, -85.74], P< 0.01), total bilirubin (MD: - 0.73, 95% CI [-1.32, -0.15], P= 0.01) and serum cholesterol (MD: -1.97, 95% CI [-3.64, -0.31], P= 0.02) were observed with IBATIs. No difference was observed in any treatment-emergent adverse events (TEAs) (RR: 1.02, 95% CI [1.12, 0.93], P= 0.71), TEAs leading to drug discontinuation (1.03, 95% CI [5.56, 0.19], P= 0.97], any serious TEAs, and liver-related TEAs.
Discussion: IBATIs reduced the Itch (ObsRo) scores, serum bile acids, total bilirubin, and cholesterol with tolerable safety profiles, indicating their promising potential in managing inherited cholestatic liver disorders.

Figure: Forest plot of Primary outcomes:
(A) Itch(ObsRo) score (B) proportion of patients achieving equal to or great than 1 point reduction in itch (ObsRo) score. MD: mean difference, CI: confidence interval.
(A) Itch(ObsRo) score (B) proportion of patients achieving equal to or great than 1 point reduction in itch (ObsRo) score. MD: mean difference, CI: confidence interval.
Disclosures:
Muhammad Imran indicated no relevant financial relationships.
Ahmed Ibrahim indicated no relevant financial relationships.
Mohamed Elnaggar indicated no relevant financial relationships.
Shujaat Ali indicated no relevant financial relationships.
Areeba Mehmood indicated no relevant financial relationships.
Saba Khalil indicated no relevant financial relationships.
Hadia Khan indicated no relevant financial relationships.
Ahmed Bostamy Elsnhory indicated no relevant financial relationships.
Omar Saab indicated no relevant financial relationships.
Mohamed Abuelazm indicated no relevant financial relationships.
Muhammad Imran, MBBS1, Ahmed A.. Ibrahim, MBBch2, Mohamed Elnaggar, MD3, Shujaat Ali, MBBS1, Areeba Mehmood, MBBS4, Saba Khalil, MBBS5, Hadia Khan, MBBS1, Ahmed Bostamy Elsnhory, MBBCh6, Omar Saab, MD7, Mohamed Abuelazm, MD8. P2935 - Efficacy and Safety of Ileal Bile Acid Transport Inhibitors in Inherited Cholestatic Liver Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
