Monday Poster Session
Category: Liver
P2984 - Rivaroxaban Goes Rogue: A Case Report on Drug-Induced Liver Injury
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- MT
Mahnoor Tariq, MBBS
Ziauddin University
Willow Grove, PA
Presenting Author(s)
Huzaif Taufiq, MD1, Mahnoor Tariq, MBBS2, Kashif Tufail, MD3
1Jefferson Torresdale Hospital, Willow Grove, PA; 2Ziauddin University, Willow Grove, PA; 3Jefferson Torresdale Hospital, Philadelphia, PA
Introduction: Rivaroxaban, an oral, direct factor Xa inhibitor, is frequently used for the prevention and treatment of thromboembolic disorders. Rivaroxaban has been associated with mild liver enzyme elevation during treatment, and with rare instances, clinically apparent liver injury with jaundice. We present a 66-year-old female with a past medical history of hypertension, and hyperlipidemia, who developed cholestatic hepatitis which resolved with cessation of rivaroxaban
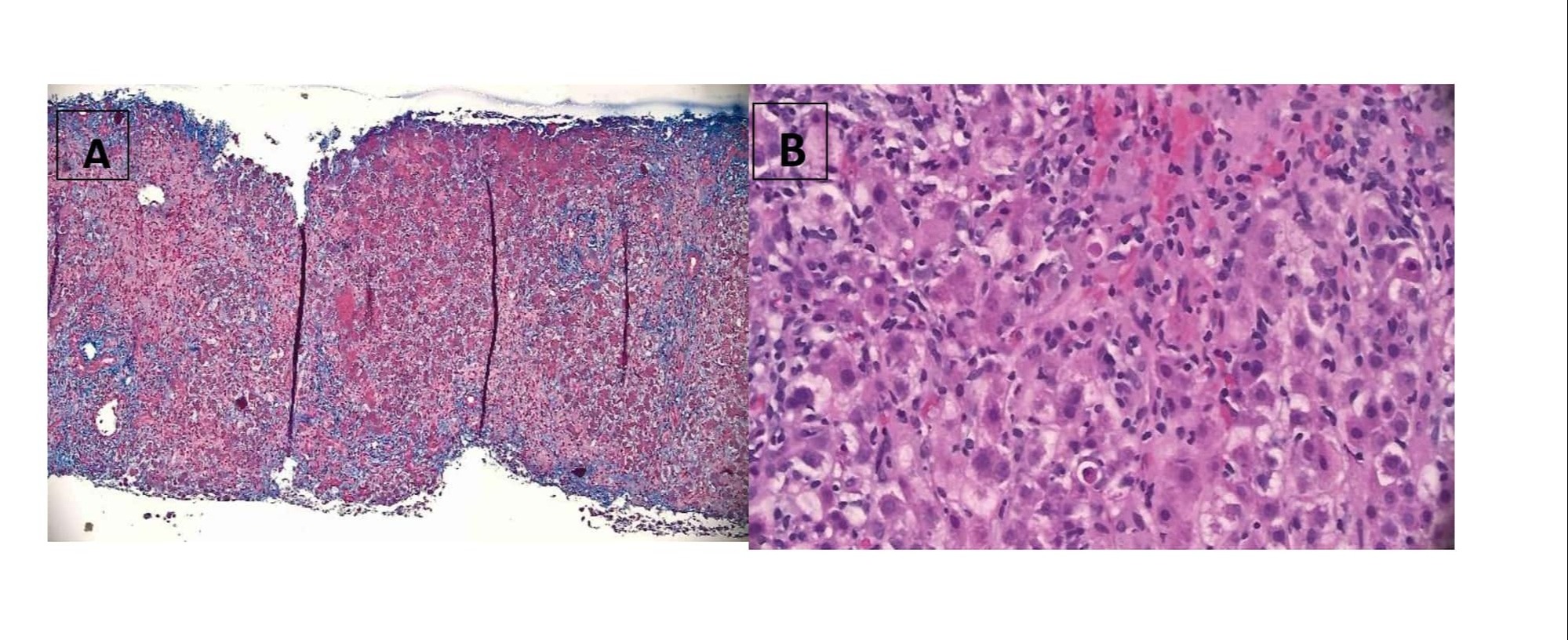
Case Description/Methods: The patient is a 66-year-old female who presented with shortness of breath and was subsequently diagnosed with acute pulmonary embolism and was started on rivaroxaban upon discharge. The patient was admitted four months later with nausea, vomiting, dizziness and jaundice for several days. Pertinent labs revealed acute liver failure with ALT 1068, AST 1825, ALP 310, INR 5.27, total bilirubin 13.9, AFP 79, Hep B/C, CMV, EBV, HSV, ANA, ASMA were negative, but grey zone for Hep A IgM Ab. CT abdomen and ultrasound imaging of liver revealed no abnormal findings. MRCP showed mild periportal edema and trace upper abdominal ascites. The plan was to evaluate the patient for a liver transplant. Patient's transaminase levels and INR improved, and transplant evaluation was therefore aborted. The patient was diagnosed with Hep A and was discharged with outpatient gastroenterology follow-up. The patient was readmitted four weeks later for persistently elevated liver enzymes and new onset jaundice. The patient underwent an urgent liver biopsy, which showed cholestatic hepatitis with severe activity, hepatocellular and canalicular cholestasis, feathery degeneration of hepatocytes with Mallory bodies, and no increase in fibrosis. These findings were consistent with drug-induced liver injury. At this point, rivaroxaban was discontinued and switched to apixaban. On follow up, liver enzymes continued to improve and eventually reached baseline levels
Discussion: Rivaroxaban is used for various clinical conditions. In our case, treatment with rivaroxaban was associated with severe liver injury. Early identification and cessation are key for the management of rivaroxaban-induced liver injury. The literature review demonstrates most patients have a favorable outcome, but a small proportion may progress to liver failure and death. Although rare, we believe hepatotoxicity should be discussed and monitored as a potential adverse reaction when commencing rivaroxaban, even if a patient has previously tolerated a drug of the same class
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Huzaif Taufiq, MD1, Mahnoor Tariq, MBBS2, Kashif Tufail, MD3. P2984 - Rivaroxaban Goes Rogue: A Case Report on Drug-Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Jefferson Torresdale Hospital, Willow Grove, PA; 2Ziauddin University, Willow Grove, PA; 3Jefferson Torresdale Hospital, Philadelphia, PA
Introduction: Rivaroxaban, an oral, direct factor Xa inhibitor, is frequently used for the prevention and treatment of thromboembolic disorders. Rivaroxaban has been associated with mild liver enzyme elevation during treatment, and with rare instances, clinically apparent liver injury with jaundice. We present a 66-year-old female with a past medical history of hypertension, and hyperlipidemia, who developed cholestatic hepatitis which resolved with cessation of rivaroxaban
Case Description/Methods: The patient is a 66-year-old female who presented with shortness of breath and was subsequently diagnosed with acute pulmonary embolism and was started on rivaroxaban upon discharge. The patient was admitted four months later with nausea, vomiting, dizziness and jaundice for several days. Pertinent labs revealed acute liver failure with ALT 1068, AST 1825, ALP 310, INR 5.27, total bilirubin 13.9, AFP 79, Hep B/C, CMV, EBV, HSV, ANA, ASMA were negative, but grey zone for Hep A IgM Ab. CT abdomen and ultrasound imaging of liver revealed no abnormal findings. MRCP showed mild periportal edema and trace upper abdominal ascites. The plan was to evaluate the patient for a liver transplant. Patient's transaminase levels and INR improved, and transplant evaluation was therefore aborted. The patient was diagnosed with Hep A and was discharged with outpatient gastroenterology follow-up. The patient was readmitted four weeks later for persistently elevated liver enzymes and new onset jaundice. The patient underwent an urgent liver biopsy, which showed cholestatic hepatitis with severe activity, hepatocellular and canalicular cholestasis, feathery degeneration of hepatocytes with Mallory bodies, and no increase in fibrosis. These findings were consistent with drug-induced liver injury. At this point, rivaroxaban was discontinued and switched to apixaban. On follow up, liver enzymes continued to improve and eventually reached baseline levels
Discussion: Rivaroxaban is used for various clinical conditions. In our case, treatment with rivaroxaban was associated with severe liver injury. Early identification and cessation are key for the management of rivaroxaban-induced liver injury. The literature review demonstrates most patients have a favorable outcome, but a small proportion may progress to liver failure and death. Although rare, we believe hepatotoxicity should be discussed and monitored as a potential adverse reaction when commencing rivaroxaban, even if a patient has previously tolerated a drug of the same class

Figure: A&B: Liver biopsy showing findings consistent with drug induced liver injury
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Huzaif Taufiq indicated no relevant financial relationships.
Mahnoor Tariq indicated no relevant financial relationships.
Kashif Tufail indicated no relevant financial relationships.
Huzaif Taufiq, MD1, Mahnoor Tariq, MBBS2, Kashif Tufail, MD3. P2984 - Rivaroxaban Goes Rogue: A Case Report on Drug-Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
