Monday Poster Session
Category: Liver
P2988 - Herb Hazard: Ashwagandha Induced Liver Injury
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Rund Nimri, MD
Jordan University of Science and Technology
Irbid, Irbid, Jordan
Presenting Author(s)
Rund Nimri, MD1, Faisal Nimri, MD2, Taher Jamali, MD2, Adarsh K. Varma, MD3, Syed-Mohammed Jafri, MD3
1Jordan University of Science and Technology, Irbid, Irbid, Jordan; 2Henry Ford Hospital, Detroit, MI; 3Henry Ford Health, Detroit, MI
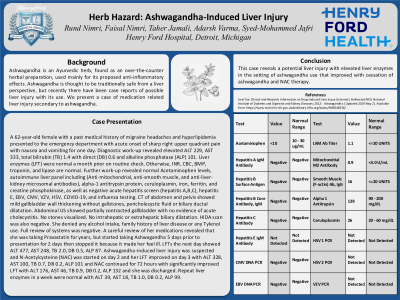
Introduction: We present a case of medication related liver injury secondary to ashwagandha. Ashwagandha is an Ayurvedic herb, found as an over-the-counter herbal preparation, used mainly for its proposed anti-inflammatory effects. Ashwagandha is thought to be safe from a liver perspective, but recently there have been case reports of possible liver injury with its use.
Case Description/Methods: A 62-year-old female with a past medical history of migraine headaches, and hyperlipidemia, presented to the ED with acute onset of sharp right upper quadrant pain with nausea and vomiting for one day. Diagnostic work-up revealed elevated ALT 229, AST 333, total bilirubin (TB) 1.4 with direct (DB) 0.6, and alkaline phosphatase (ALP) 101. Liver enzymes (LFT) were normal a month prior on routine check. Otherwise, INR, CBC, BMP, troponin, and lipase are normal. Further work-up revealed normal Acetaminophen levels, autoimmune liver panel including (Anti-mitochondrial, anti-smooth muscle, and anti-liver-kidney microsomal antibodies), alpha-1 antitrypsin protein, ceruloplasmin, iron, ferritin, and creatine phosphokinase, as well as negative acute hepatitis screen (hepatitis A,B,C), hepatitis E, EBV, CMV, VZV, HSV, COVID-19, and influenza testing. CT of abdomen and pelvis showed mild gallbladder wall thickening without gallstones, pericholecystic fluid or biliary ductal dilatation. Abdominal US showed partially contracted gallbladder with no evidence of acute cholecystitis. No stones visualized. No intrahepatic or extrahepatic biliary dilatation. HIDA scan was also negative. She denied any alcohol intake, family history of liver disease or any Tylenol use. Full review of systems was negative. A careful review of her medications revealed that she was taking Pravastatin for years, but started taking Ashwagandha 5 days prior to presentation for 2 days then stopped it because it made her feel ill. LFTs the next day showed ALT 477, AST 248, TB 2.0, DB 0.5, ALP 87. Ashwagandha-induced liver injury was suspected and N-Acetylcysteine (NAC) was started on day 2 and her LFT improved on day 3 with ALT 328, AST 100, TB 0.7, DB 0.2, ALP 101 and NAC continued for 72 hours with significantly improved LFT with ALT 176, AST 46, TB 0.9, DB 0.2, ALP 132 and she was discharged. Repeat liver enzymes in a week were normal with ALT 39, AST 18, TB 1.0, DB 0.2, ALP 99.
Discussion: This case reveals a potential liver injury with elevated liver enzymes in the setting of ashwagandha use that improved with cessation of ashwagandha and NAC therapy.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rund Nimri, MD1, Faisal Nimri, MD2, Taher Jamali, MD2, Adarsh K. Varma, MD3, Syed-Mohammed Jafri, MD3. P2988 - Herb Hazard: Ashwagandha Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Jordan University of Science and Technology, Irbid, Irbid, Jordan; 2Henry Ford Hospital, Detroit, MI; 3Henry Ford Health, Detroit, MI
Introduction: We present a case of medication related liver injury secondary to ashwagandha. Ashwagandha is an Ayurvedic herb, found as an over-the-counter herbal preparation, used mainly for its proposed anti-inflammatory effects. Ashwagandha is thought to be safe from a liver perspective, but recently there have been case reports of possible liver injury with its use.
Case Description/Methods: A 62-year-old female with a past medical history of migraine headaches, and hyperlipidemia, presented to the ED with acute onset of sharp right upper quadrant pain with nausea and vomiting for one day. Diagnostic work-up revealed elevated ALT 229, AST 333, total bilirubin (TB) 1.4 with direct (DB) 0.6, and alkaline phosphatase (ALP) 101. Liver enzymes (LFT) were normal a month prior on routine check. Otherwise, INR, CBC, BMP, troponin, and lipase are normal. Further work-up revealed normal Acetaminophen levels, autoimmune liver panel including (Anti-mitochondrial, anti-smooth muscle, and anti-liver-kidney microsomal antibodies), alpha-1 antitrypsin protein, ceruloplasmin, iron, ferritin, and creatine phosphokinase, as well as negative acute hepatitis screen (hepatitis A,B,C), hepatitis E, EBV, CMV, VZV, HSV, COVID-19, and influenza testing. CT of abdomen and pelvis showed mild gallbladder wall thickening without gallstones, pericholecystic fluid or biliary ductal dilatation. Abdominal US showed partially contracted gallbladder with no evidence of acute cholecystitis. No stones visualized. No intrahepatic or extrahepatic biliary dilatation. HIDA scan was also negative. She denied any alcohol intake, family history of liver disease or any Tylenol use. Full review of systems was negative. A careful review of her medications revealed that she was taking Pravastatin for years, but started taking Ashwagandha 5 days prior to presentation for 2 days then stopped it because it made her feel ill. LFTs the next day showed ALT 477, AST 248, TB 2.0, DB 0.5, ALP 87. Ashwagandha-induced liver injury was suspected and N-Acetylcysteine (NAC) was started on day 2 and her LFT improved on day 3 with ALT 328, AST 100, TB 0.7, DB 0.2, ALP 101 and NAC continued for 72 hours with significantly improved LFT with ALT 176, AST 46, TB 0.9, DB 0.2, ALP 132 and she was discharged. Repeat liver enzymes in a week were normal with ALT 39, AST 18, TB 1.0, DB 0.2, ALP 99.
Discussion: This case reveals a potential liver injury with elevated liver enzymes in the setting of ashwagandha use that improved with cessation of ashwagandha and NAC therapy.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rund Nimri indicated no relevant financial relationships.
Faisal Nimri indicated no relevant financial relationships.
Taher Jamali indicated no relevant financial relationships.
Adarsh Varma indicated no relevant financial relationships.
Syed-Mohammed Jafri: Gilead, Takeda, Abbvie, Intercept, VectivBio – Advisor or Review Panel Member, Speakers Bureau.
Rund Nimri, MD1, Faisal Nimri, MD2, Taher Jamali, MD2, Adarsh K. Varma, MD3, Syed-Mohammed Jafri, MD3. P2988 - Herb Hazard: Ashwagandha Induced Liver Injury, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
