Monday Poster Session
Category: Liver
P3046 - Drug-Induced Liver Injury Secondary to Recent Ozempic use
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Hobie L. Hughes, DO
Jefferson Health
Daphne, AL
Presenting Author(s)
Hobie Hughes, DO1, Lauren Healey, DO2, Joshua Soliman, DO3, Lucy Joo, DO4
1Jefferson Health, Williamstown, NJ; 2USA Health, University of South Alabama, Williamstown, NJ; 3Jefferson Health, Washington Township, NJ; 4Jefferson Health, Cherry Hill, NJ
Introduction: Drug-induced liver injury (DILI) is commonly seen, yet it remains an exceedingly challenging disorder in terms of both diagnosis and management given its wide-ranging presentation, potential for multiple offending agents, and a lack of objective diagnostic testing. Many classes of prescription and over the counter medications, as well as supplements, have been known to cause DILI with most cases being mild and quickly improve once the offending agent is discontinued. The immediate recognition and discontinuation of potential offending agents is an important first step during any liver disease workup to prevent potential progression to chronic liver disease or acute liver failure.
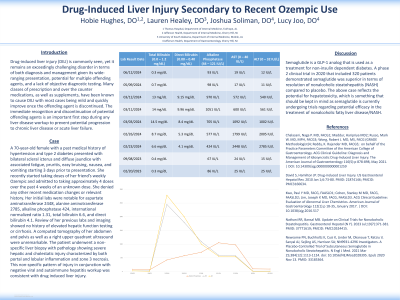
Case Description/Methods: A 70-year-old female with a past medical history of hypertension and type 2 diabetes presented for evaluation of bilateral scleral icterus and diffuse jaundice with associated fatigue, pruritis, easy bruising, nausea, and vomiting starting 3 days prior to presentation. She stated that she recently started taking doses of her friend’s weekly Ozempic and admitted to taking approximately 4 doses over the past 4 weeks of an unknown dose. She denied any other recent medication changes or relevant history. Her initial labs were notable for aspartate aminotransferase 2448, alanine aminotransferase 2785, alkaline phosphatase 424, international normalized ratio 1.31, total bilirubin 6.6, and direct bilirubin 4.1. Review of her previous labs and imaging showed no history of elevated hepatic function testing or cirrhosis. A computed tomography of her abdomen and pelvis as well as a right upper quadrant ultrasound were unremarkable. The patient underwent a non-specific liver biopsy with pathology showing severe hepatic and cholestatic injury characterized by both portal and lobular inflammation and zone 3 necrosis. This non-specific pattern of injury in conjunction with negative viral and autoimmune hepatitis workup was consistent with drug induced liver injury.
Discussion: Semaglutide is a GLP-1 analog that is used as a treatment for non-insulin dependent diabetes. A phase 2 clinical trial in 2020 that included 320 patients demonstrated semaglutide was superior in terms of resolution of nonalcoholic steatohepatitis (NASH) compared to placebo. The above case reflects the potential for hepatotoxicity, which is something that should be kept in mind as semaglutide is currently undergoing trials regarding potential efficacy in the treatment of nonalcoholic fatty liver disease/NASH.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Hobie Hughes, DO1, Lauren Healey, DO2, Joshua Soliman, DO3, Lucy Joo, DO4. P3046 - Drug-Induced Liver Injury Secondary to Recent Ozempic use, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Jefferson Health, Williamstown, NJ; 2USA Health, University of South Alabama, Williamstown, NJ; 3Jefferson Health, Washington Township, NJ; 4Jefferson Health, Cherry Hill, NJ
Introduction: Drug-induced liver injury (DILI) is commonly seen, yet it remains an exceedingly challenging disorder in terms of both diagnosis and management given its wide-ranging presentation, potential for multiple offending agents, and a lack of objective diagnostic testing. Many classes of prescription and over the counter medications, as well as supplements, have been known to cause DILI with most cases being mild and quickly improve once the offending agent is discontinued. The immediate recognition and discontinuation of potential offending agents is an important first step during any liver disease workup to prevent potential progression to chronic liver disease or acute liver failure.
Case Description/Methods: A 70-year-old female with a past medical history of hypertension and type 2 diabetes presented for evaluation of bilateral scleral icterus and diffuse jaundice with associated fatigue, pruritis, easy bruising, nausea, and vomiting starting 3 days prior to presentation. She stated that she recently started taking doses of her friend’s weekly Ozempic and admitted to taking approximately 4 doses over the past 4 weeks of an unknown dose. She denied any other recent medication changes or relevant history. Her initial labs were notable for aspartate aminotransferase 2448, alanine aminotransferase 2785, alkaline phosphatase 424, international normalized ratio 1.31, total bilirubin 6.6, and direct bilirubin 4.1. Review of her previous labs and imaging showed no history of elevated hepatic function testing or cirrhosis. A computed tomography of her abdomen and pelvis as well as a right upper quadrant ultrasound were unremarkable. The patient underwent a non-specific liver biopsy with pathology showing severe hepatic and cholestatic injury characterized by both portal and lobular inflammation and zone 3 necrosis. This non-specific pattern of injury in conjunction with negative viral and autoimmune hepatitis workup was consistent with drug induced liver injury.
Discussion: Semaglutide is a GLP-1 analog that is used as a treatment for non-insulin dependent diabetes. A phase 2 clinical trial in 2020 that included 320 patients demonstrated semaglutide was superior in terms of resolution of nonalcoholic steatohepatitis (NASH) compared to placebo. The above case reflects the potential for hepatotoxicity, which is something that should be kept in mind as semaglutide is currently undergoing trials regarding potential efficacy in the treatment of nonalcoholic fatty liver disease/NASH.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Hobie Hughes indicated no relevant financial relationships.
Lauren Healey indicated no relevant financial relationships.
Joshua Soliman indicated no relevant financial relationships.
Lucy Joo indicated no relevant financial relationships.
Hobie Hughes, DO1, Lauren Healey, DO2, Joshua Soliman, DO3, Lucy Joo, DO4. P3046 - Drug-Induced Liver Injury Secondary to Recent Ozempic use, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

