Monday Poster Session
Category: Liver
P3074 - The Importance of Genetic Testing in a Case of Oral Contraceptive Induced Jaundice as First Manifestation of Benign Recurrent Intrahepatic Cholestasis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- NS
Nishali Shah, MD
Robert Wood Johnson Medical School, Rutgers University
New Brunswick, NJ
Presenting Author(s)
Award: Presidential Poster Award
Nishali Shah, MD, Meghana Ghattu, MD, Alexander T. Lalos, MD, Rachel Hudacko, MD
Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ
Introduction: Benign recurrent intrahepatic cholestasis (BRIC) is a rare cause of episodic and non-progressive cholestatic liver injury. We report a case of BRIC that was triggered by oral contraceptive pills.
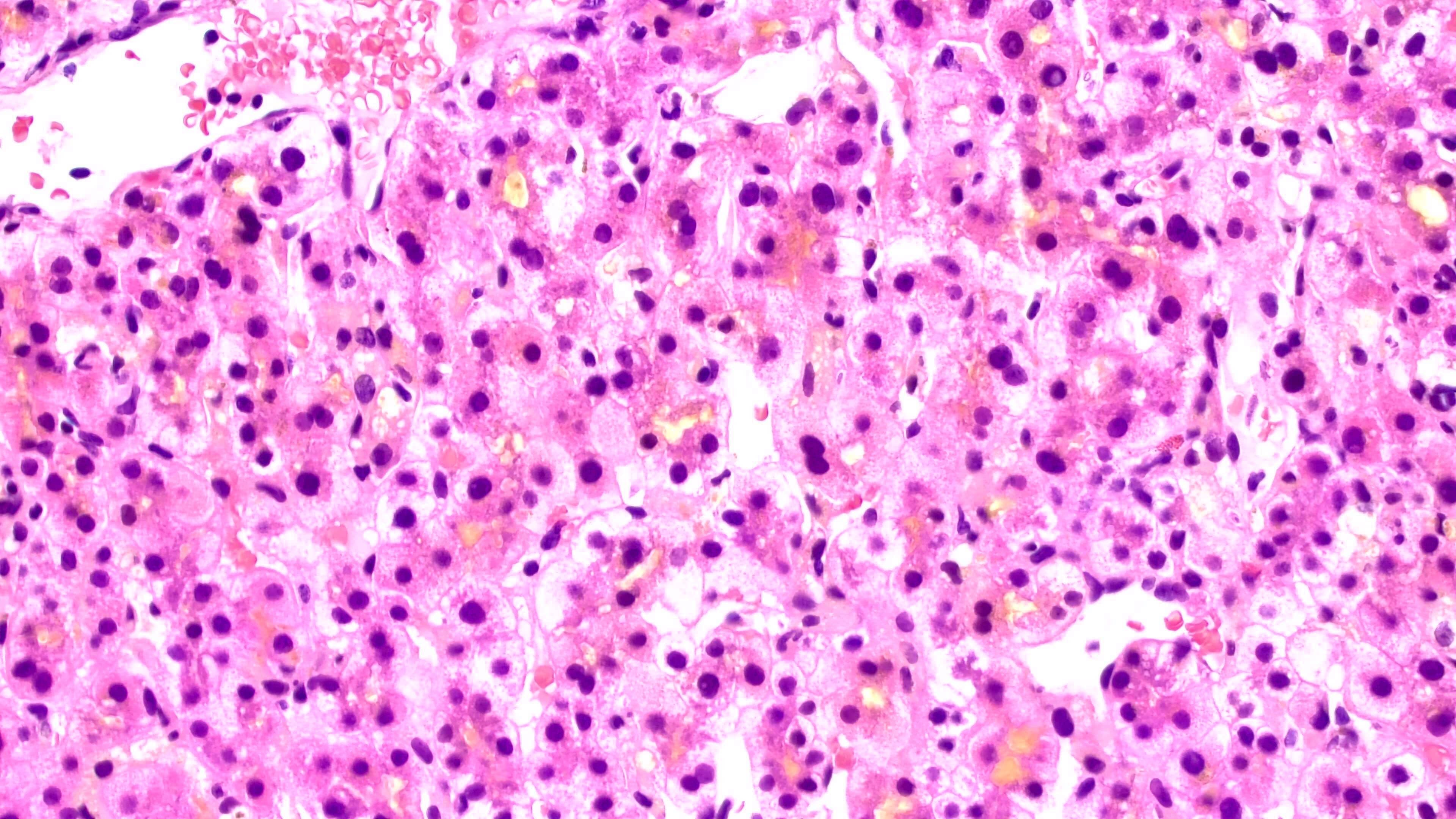
Case Description/Methods: A 19-year-old female with no medical history presented with one-week of jaundice, pruritus, and abdominal pain. Six months prior she had started estrogen containing oral contraceptive pills (OCPs). Labs revealed markedly elevated bilirubin with elevated transaminases and alkaline phosphatase, and normal gamma-glutamyltransferase (GGT). Additional serologic workup was unremarkable and all abdominal imaging was normal. Liver biopsy revealed diffuse cholestasis with few foci of lobular inflammation (Figure 1). She was diagnosed with a cholestatic drug-induced liver injury secondary to OCPs. The normal GGT raised the possibility of BRIC. Genetic testing revealed a heterozygous variant of the ABCB11 gene known to be associated with BRIC. OCPs were discontinued and she was treated with ursodiol and rifampicin. Symptoms and lab abnormalities resolved over the following two months. Months later the patient developed a second episode of jaundice that resolved spontaneously.
Discussion: BRIC presents as recurrent episodes of cholestasis without progressive liver damage which can last weeks to months.¹ During episodes, patients have elevated transaminases and bilirubin but notably normal GGT levels.² BRIC in most cases is now known to be associated with mutations in the ABCB11 gene, which encodes for the bile acid secretion pump protein. Patients with an underlying mutation often have a trigger associated with development of cholestasis such as infection or estrogen exposure, including pregnancy.³ Increased levels of estrogen decrease fluidity of hepatocyte lipid membranes and disrupt Na+/K+ ATPase and ATP-dependent bile acid secretion pump activity.³ In this case, a patient with an underlying ABCB11 mutation developed cholestasis with exposure to estrogen. We aim to heighten awareness of BRIC and the role of genetic testing in patients with cholestatic liver injury after OCP initiation, as it may guide risk of ongoing hormonal OCP use or pregnancy.
Disclosures:
Nishali Shah, MD, Meghana Ghattu, MD, Alexander T. Lalos, MD, Rachel Hudacko, MD. P3074 - The Importance of Genetic Testing in a Case of Oral Contraceptive Induced Jaundice as First Manifestation of Benign Recurrent Intrahepatic Cholestasis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Nishali Shah, MD, Meghana Ghattu, MD, Alexander T. Lalos, MD, Rachel Hudacko, MD
Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ
Introduction: Benign recurrent intrahepatic cholestasis (BRIC) is a rare cause of episodic and non-progressive cholestatic liver injury. We report a case of BRIC that was triggered by oral contraceptive pills.
Case Description/Methods: A 19-year-old female with no medical history presented with one-week of jaundice, pruritus, and abdominal pain. Six months prior she had started estrogen containing oral contraceptive pills (OCPs). Labs revealed markedly elevated bilirubin with elevated transaminases and alkaline phosphatase, and normal gamma-glutamyltransferase (GGT). Additional serologic workup was unremarkable and all abdominal imaging was normal. Liver biopsy revealed diffuse cholestasis with few foci of lobular inflammation (Figure 1). She was diagnosed with a cholestatic drug-induced liver injury secondary to OCPs. The normal GGT raised the possibility of BRIC. Genetic testing revealed a heterozygous variant of the ABCB11 gene known to be associated with BRIC. OCPs were discontinued and she was treated with ursodiol and rifampicin. Symptoms and lab abnormalities resolved over the following two months. Months later the patient developed a second episode of jaundice that resolved spontaneously.
Discussion: BRIC presents as recurrent episodes of cholestasis without progressive liver damage which can last weeks to months.¹ During episodes, patients have elevated transaminases and bilirubin but notably normal GGT levels.² BRIC in most cases is now known to be associated with mutations in the ABCB11 gene, which encodes for the bile acid secretion pump protein. Patients with an underlying mutation often have a trigger associated with development of cholestasis such as infection or estrogen exposure, including pregnancy.³ Increased levels of estrogen decrease fluidity of hepatocyte lipid membranes and disrupt Na+/K+ ATPase and ATP-dependent bile acid secretion pump activity.³ In this case, a patient with an underlying ABCB11 mutation developed cholestasis with exposure to estrogen. We aim to heighten awareness of BRIC and the role of genetic testing in patients with cholestatic liver injury after OCP initiation, as it may guide risk of ongoing hormonal OCP use or pregnancy.
- van Mil et al. “BRIC T2 is caused by mutations in ABCB11.” Gastro. 127,2 (2004): 379-84.
- Geladari EV et al.: “BRIC: Where Are We Now? Gastro Insights 15,1 (2024):156-167.
- Schreiber, AJ et al.. “Estrogen-induced cholestasis: clues to pathogenesis and treatment.” Hepatology 3,4 (1983): 607-13.

Figure: Figure 1. Hematoxylin and Eosin (H&E) staining of liver demonstrating diffuse cholestasis with few foci of lobular inflammation.
Disclosures:
Nishali Shah indicated no relevant financial relationships.
Meghana Ghattu indicated no relevant financial relationships.
Alexander Lalos indicated no relevant financial relationships.
Rachel Hudacko indicated no relevant financial relationships.
Nishali Shah, MD, Meghana Ghattu, MD, Alexander T. Lalos, MD, Rachel Hudacko, MD. P3074 - The Importance of Genetic Testing in a Case of Oral Contraceptive Induced Jaundice as First Manifestation of Benign Recurrent Intrahepatic Cholestasis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


