Monday Poster Session
Category: Liver
P3130 - Challenges in Managing Hepatitis B Reactivation in an HIV-Positive Patient on Dialysis for End-Stage Renal Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Kanza Shamim, MD
Nassau University Medical Center
Merrick, NY
Presenting Author(s)
Kanza Shamim, MD1, Christine Flores, MD2, Kim Zae, MD2, Hamid Reza Pahlevan Sabbagh, MD2, Shadab Ahmed, MD2
1Nassau University Medical Center, Merrick, NY; 2Nassau University Medical Center, East Meadow, NY
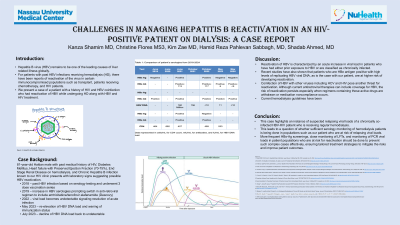
Introduction: Hepatitis B virus (HBV) remains to be one of the leading causes of liver related illness globally. Worldwide 296 million people are seropositive for HBV infection ¹. Between 880,000 and 1.89 million individuals are living with chronic hepatitis B in the US according to 2021 data ¹. It has been established that patients who are receiving HD regularly are at increased risk for contracting HBV² due to their continued contact with blood products, multiple hospital visits, and chronic vascular access.
Case Description/Methods: 81 year old male with a past medical history of HIV, Hepatitis B and ESRD, DM, HFpEF, and ESRD on maintenance HD since 2018. Patient was initially seropositive for anti-HBc, and seronegative for HBsAg and HCV. Received HBV vaccination in 2019. Patient had recurrent admissions due to comorbidities. In June 2021 the patient was seropositive for HBsAg, anti-HBs, anti-HBc, HBeAg, anti-HBe and HBV DNA was 160M IU/mL with CD4 count 427 cells/mL. Patient was started on emtricitabine/tenofovir alafenamide (Descovy). In October 2022 Patient had undetectable HBV DNA with CD4: 499 cells/mL. In May 2023 patient had detectable HBV DNA: 11 copies/mL, anti-HBs: 6 mIU/mL with seronegative HBsAg. In July 2023 patient had undetectable HBV DNA, seronegative for HBsAg. In May 2024 patient was readmitted for atrial flutter, required HD. He was seronegative for HBsAg, anti-HBc positive, with undetectable HBV DNA, seropositive for HBeAg and anti-HBe. Current regimen includes Prezista, ritonavir, TDF, dolutegravir, and lamivudine.
Discussion: Due to risk of developing hepatic failure with continued HBV reactivation, prophylaxis treatment of HbcAg in immunosuppressed patients as early as possible³,⁴ . The American Gastroenterology Association recommends prophylaxis antiretroviral treatment for those deemed high or moderate risk of reactivation ⁵. Treatment should continue for at least 6 months after discontinuation of immunosuppressive medications³.Nucleoside analogues like lamivudine, tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), and entecavir, have shown to be the most effective prophylaxis³ . Patients who receive chronic hemodialysis must undergo routine serologic testing every 6 months to determine their current viral status. These laboratory tests include HBs antigen, anti-HBs, total anti-HBc and current viral load. All patients susceptible to HBV infection should receive the 3 dose vaccination series prior to starting dialysis treatment ⁶.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Kanza Shamim, MD1, Christine Flores, MD2, Kim Zae, MD2, Hamid Reza Pahlevan Sabbagh, MD2, Shadab Ahmed, MD2. P3130 - Challenges in Managing Hepatitis B Reactivation in an HIV-Positive Patient on Dialysis for End-Stage Renal Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Nassau University Medical Center, Merrick, NY; 2Nassau University Medical Center, East Meadow, NY
Introduction: Hepatitis B virus (HBV) remains to be one of the leading causes of liver related illness globally. Worldwide 296 million people are seropositive for HBV infection ¹. Between 880,000 and 1.89 million individuals are living with chronic hepatitis B in the US according to 2021 data ¹. It has been established that patients who are receiving HD regularly are at increased risk for contracting HBV² due to their continued contact with blood products, multiple hospital visits, and chronic vascular access.
Case Description/Methods: 81 year old male with a past medical history of HIV, Hepatitis B and ESRD, DM, HFpEF, and ESRD on maintenance HD since 2018. Patient was initially seropositive for anti-HBc, and seronegative for HBsAg and HCV. Received HBV vaccination in 2019. Patient had recurrent admissions due to comorbidities. In June 2021 the patient was seropositive for HBsAg, anti-HBs, anti-HBc, HBeAg, anti-HBe and HBV DNA was 160M IU/mL with CD4 count 427 cells/mL. Patient was started on emtricitabine/tenofovir alafenamide (Descovy). In October 2022 Patient had undetectable HBV DNA with CD4: 499 cells/mL. In May 2023 patient had detectable HBV DNA: 11 copies/mL, anti-HBs: 6 mIU/mL with seronegative HBsAg. In July 2023 patient had undetectable HBV DNA, seronegative for HBsAg. In May 2024 patient was readmitted for atrial flutter, required HD. He was seronegative for HBsAg, anti-HBc positive, with undetectable HBV DNA, seropositive for HBeAg and anti-HBe. Current regimen includes Prezista, ritonavir, TDF, dolutegravir, and lamivudine.
Discussion: Due to risk of developing hepatic failure with continued HBV reactivation, prophylaxis treatment of HbcAg in immunosuppressed patients as early as possible³,⁴ . The American Gastroenterology Association recommends prophylaxis antiretroviral treatment for those deemed high or moderate risk of reactivation ⁵. Treatment should continue for at least 6 months after discontinuation of immunosuppressive medications³.Nucleoside analogues like lamivudine, tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), and entecavir, have shown to be the most effective prophylaxis³ . Patients who receive chronic hemodialysis must undergo routine serologic testing every 6 months to determine their current viral status. These laboratory tests include HBs antigen, anti-HBs, total anti-HBc and current viral load. All patients susceptible to HBV infection should receive the 3 dose vaccination series prior to starting dialysis treatment ⁶.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Kanza Shamim indicated no relevant financial relationships.
Christine Flores indicated no relevant financial relationships.
Kim Zae indicated no relevant financial relationships.
Hamid Reza Pahlevan Sabbagh indicated no relevant financial relationships.
Shadab Ahmed indicated no relevant financial relationships.
Kanza Shamim, MD1, Christine Flores, MD2, Kim Zae, MD2, Hamid Reza Pahlevan Sabbagh, MD2, Shadab Ahmed, MD2. P3130 - Challenges in Managing Hepatitis B Reactivation in an HIV-Positive Patient on Dialysis for End-Stage Renal Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
