Monday Poster Session
Category: Small Intestine
P3186 - Tissue Transglutaminase (TTG) Assay: Hurdles to a No-Biopsy Approach for Diagnosing Celiac Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Rangesh Modi, MD
University of Chicago Medicine
Chicago, IL
Presenting Author(s)
Rangesh Modi, MD1, Dejan Micic, MD2, Carol E. Semrad, MD2
1University of Chicago Medicine, Chicago, IL; 2University of Chicago Medical Center, Chicago, IL
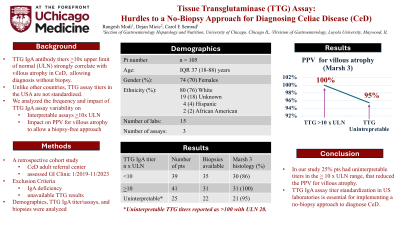
Introduction: Tissue transglutaminase (TTG) IgA antibody levels > 10 times the upper limit of normal (ULN) have a high correlation with villous atrophy (98-100% PPV) in children (J Pediatr Gastroenterol Nutr. 2020;70:141) and adults (Gastroenterology 2024;166:620) with celiac disease, with the potential to forgo duodenal biopsy for diagnosis. In the United States, TTG assays are variable in performance and titer reporting and not standardized. We analyzed how often TTG IgA assays allowed for the interpretation of a >10 times the ULN result to forgo biopsy for diagnosis.
Methods: A retrospective cohort study utilizing the University of Chicago patient database identified over 400 patients referred with a diagnosis of celiac disease to the adult clinic between 1/1/2019 and 11/1/2023. We excluded patients with IgA deficiency or TTG assay results that were unavailable upon diagnosis. We analyzed demographic data, TTG IgA titers/reference ranges, and pathology results. The primary outcome was the number of TTG IgA titers that allowed interpretation in > 10 times the ULN range. Descriptive statistics were used to analyze data.
Results: To date, we analyzed data from 105 patients. The median age was 33 (IQR: 23-50) years. Seventy-four (70%) were women and 80 (76%) were White. Fifteen laboratories utilized three manufacturer assays to report TTG IgA levels. In 25 of 105 (24%) patients, the TTG IgA assay was not titrated to a definitive number to allow interpretation for > 10 times the ULN. For all 25 patients, the assay results were reported as >100, with the ULN < 20. Biopsies were available for review in 22 of these patients; 21/22 (95%) patients had Marsh 3 lesions and 1/22 (5%) had a Marsh 1 lesion. In the remaining 80 of 105 patients with interpretable titers, 41/80 (51%) had TTG IgA levels > 10 times the ULN. Duodenal biopsies were available for review in 31 of these patients and all (100%) had Marsh 3 lesions.
Discussion: A no-biopsy approach, when appropriate, is cost-effective and spares patients from undergoing invasive endoscopy. Our results show that a quarter of our patients had TTG assays that were not titered to allow interpretation of >10 times the ULN to forgo duodenal biopsy. To integrate a no-biopsy strategy for diagnosing celiac disease into clinical practice, it is essential to standardize the TTG IgA assays across laboratories in the United States.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rangesh Modi, MD1, Dejan Micic, MD2, Carol E. Semrad, MD2. P3186 - Tissue Transglutaminase (TTG) Assay: Hurdles to a No-Biopsy Approach for Diagnosing Celiac Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Chicago Medicine, Chicago, IL; 2University of Chicago Medical Center, Chicago, IL
Introduction: Tissue transglutaminase (TTG) IgA antibody levels > 10 times the upper limit of normal (ULN) have a high correlation with villous atrophy (98-100% PPV) in children (J Pediatr Gastroenterol Nutr. 2020;70:141) and adults (Gastroenterology 2024;166:620) with celiac disease, with the potential to forgo duodenal biopsy for diagnosis. In the United States, TTG assays are variable in performance and titer reporting and not standardized. We analyzed how often TTG IgA assays allowed for the interpretation of a >10 times the ULN result to forgo biopsy for diagnosis.
Methods: A retrospective cohort study utilizing the University of Chicago patient database identified over 400 patients referred with a diagnosis of celiac disease to the adult clinic between 1/1/2019 and 11/1/2023. We excluded patients with IgA deficiency or TTG assay results that were unavailable upon diagnosis. We analyzed demographic data, TTG IgA titers/reference ranges, and pathology results. The primary outcome was the number of TTG IgA titers that allowed interpretation in > 10 times the ULN range. Descriptive statistics were used to analyze data.
Results: To date, we analyzed data from 105 patients. The median age was 33 (IQR: 23-50) years. Seventy-four (70%) were women and 80 (76%) were White. Fifteen laboratories utilized three manufacturer assays to report TTG IgA levels. In 25 of 105 (24%) patients, the TTG IgA assay was not titrated to a definitive number to allow interpretation for > 10 times the ULN. For all 25 patients, the assay results were reported as >100, with the ULN < 20. Biopsies were available for review in 22 of these patients; 21/22 (95%) patients had Marsh 3 lesions and 1/22 (5%) had a Marsh 1 lesion. In the remaining 80 of 105 patients with interpretable titers, 41/80 (51%) had TTG IgA levels > 10 times the ULN. Duodenal biopsies were available for review in 31 of these patients and all (100%) had Marsh 3 lesions.
Discussion: A no-biopsy approach, when appropriate, is cost-effective and spares patients from undergoing invasive endoscopy. Our results show that a quarter of our patients had TTG assays that were not titered to allow interpretation of >10 times the ULN to forgo duodenal biopsy. To integrate a no-biopsy strategy for diagnosing celiac disease into clinical practice, it is essential to standardize the TTG IgA assays across laboratories in the United States.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Rangesh Modi indicated no relevant financial relationships.
Dejan Micic: Ironwood Pharmaceuticals – Advisory Committee/Board Member. Takeda Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Carol Semrad indicated no relevant financial relationships.
Rangesh Modi, MD1, Dejan Micic, MD2, Carol E. Semrad, MD2. P3186 - Tissue Transglutaminase (TTG) Assay: Hurdles to a No-Biopsy Approach for Diagnosing Celiac Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
