Monday Poster Session
Category: Small Intestine
P3200 - Pneumococcal Vaccine is Associated with Better Outcomes in Celiac Disease Patients: Insights From a Propensity-Matched Study in the United States
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Mouhand F.H Mohamed, MD, MSc
Mayo Clinic
Rochester, MN
Presenting Author(s)
Mouhand F.H. Mohamed, MD, MSc1, Osama Hamid, MD2, Azizullah Beran, MD3, Xiao Jing (Iris) Wang, MD1, Adam Bledsoe, MD1
1Mayo Clinic, Rochester, MN; 2University of Texas Southwestern Medical Center, Dallas, TX; 3Indiana University School of Medicine, Indianapolis, IN
Introduction: Pneumococcal vaccine is recommended for celiac disease (CD) patients due to increased pneumonia risk, presumably from hyposplenism. This recommendation is based on low evidence certainty. To date, it is not well established that pneumococcal vaccine leads to better outcomes in CD. This study evaluates the outcomes of the pneumonia vaccine in CD patients.
Methods: We analyzed TriNetX US Collaborative Network data, including > 100 million patient records from 64 healthcare organizations. Adults > 18 years with CD were included (January 2021 to May 2024). Identification was conducted using ICD-10 codes. Due to its single-dose regimen, we only included CD patients who received the pneumococcal vaccine 20-valent (PCV20) as a surrogate for complete pneumococcal vaccination. For the control group, we excluded all forms of pneumococcal vaccines. We did a subgroup analysis for patients < 65 years. A 1:1 propensity score matching (PSM) was done to account for common pneumonia risk factors (Table 1). We assessed all outcomes occurring 30 days or more after the vaccine was administered. The primary outcome was the risk of pneumonia. Other outcomes included hospital admissions, intensive care unit (ICU) service utilization, and acute respiratory failure (ARF). Relative risk (RR) and 95% confidence intervals (CI) were generated for comparisons.
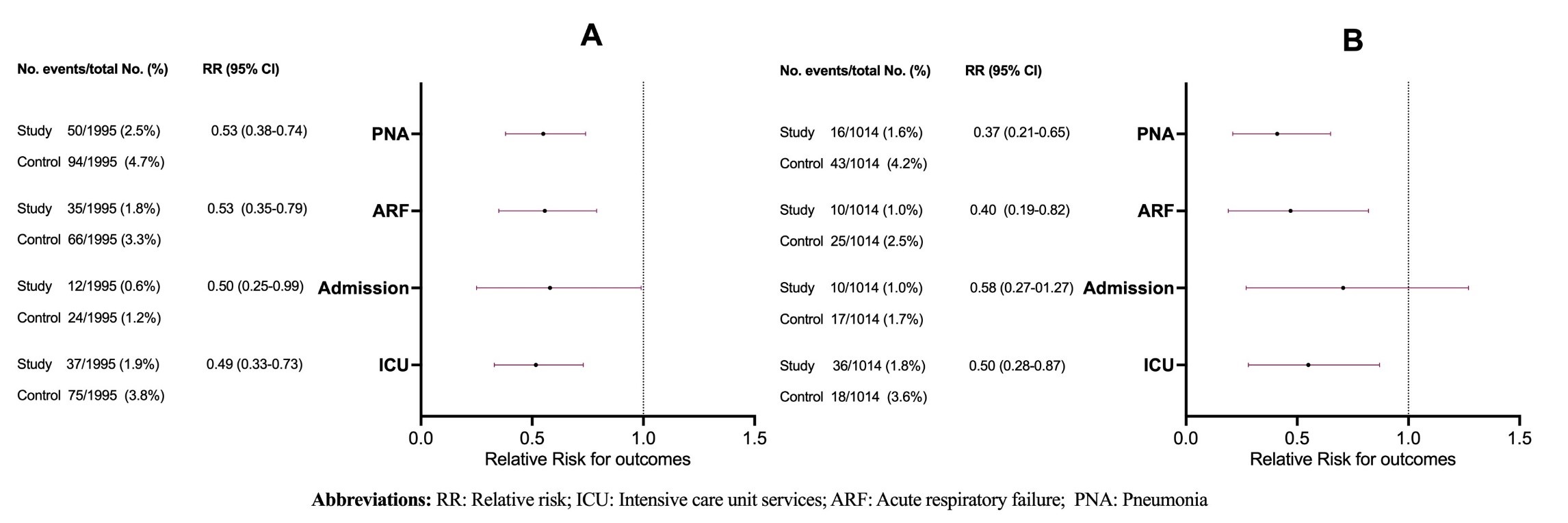
Results: After PSM, 3,990 patients were included in the analysis (1,995 in each arm) (Table 1). Compared to the control group, the vaccinated group had a significantly lower risk of pneumonia (RR 0.53 [95% CI 0.38-0.74]), ARF (RR 0.53 [95% CI 0.35-0.79]), hospital admissions (RR 0.50 [95% CI 0.25-0.99]), and ICU service utilization (RR 0.49 [95% CI 0.33-0.73]) (Figure 1). No cases of meningitis were reported in either group. For patients < 65 years old, compared to the unvaccinated subgroup, the vaccinated subgroup showed a lower risk of pneumonia (RR 0.37 [95% CI 0.21-0.65]), ARF (RR 0.40 [95% CI 0.19-0.82]), and ICU service utilization (RR 0.50 [95% CI 0.29-0.87]). Hospital admission rates were numerically lower but not significantly different in the vaccinated group (RR 0.59 [95% CI 0.27-1.27]) (Figure 1).
Discussion: This large database analysis revealed lower pneumonia risk and better health outcomes associated with pneumococcal vaccination in CD patients, strongly supporting CD's current pneumococcal vaccination guidelines. Further research is needed into the comparative effectiveness of different pneumococcal vaccine formulations.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mouhand F.H. Mohamed, MD, MSc1, Osama Hamid, MD2, Azizullah Beran, MD3, Xiao Jing (Iris) Wang, MD1, Adam Bledsoe, MD1. P3200 - Pneumococcal Vaccine is Associated with Better Outcomes in Celiac Disease Patients: Insights From a Propensity-Matched Study in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2University of Texas Southwestern Medical Center, Dallas, TX; 3Indiana University School of Medicine, Indianapolis, IN
Introduction: Pneumococcal vaccine is recommended for celiac disease (CD) patients due to increased pneumonia risk, presumably from hyposplenism. This recommendation is based on low evidence certainty. To date, it is not well established that pneumococcal vaccine leads to better outcomes in CD. This study evaluates the outcomes of the pneumonia vaccine in CD patients.
Methods: We analyzed TriNetX US Collaborative Network data, including > 100 million patient records from 64 healthcare organizations. Adults > 18 years with CD were included (January 2021 to May 2024). Identification was conducted using ICD-10 codes. Due to its single-dose regimen, we only included CD patients who received the pneumococcal vaccine 20-valent (PCV20) as a surrogate for complete pneumococcal vaccination. For the control group, we excluded all forms of pneumococcal vaccines. We did a subgroup analysis for patients < 65 years. A 1:1 propensity score matching (PSM) was done to account for common pneumonia risk factors (Table 1). We assessed all outcomes occurring 30 days or more after the vaccine was administered. The primary outcome was the risk of pneumonia. Other outcomes included hospital admissions, intensive care unit (ICU) service utilization, and acute respiratory failure (ARF). Relative risk (RR) and 95% confidence intervals (CI) were generated for comparisons.
Results: After PSM, 3,990 patients were included in the analysis (1,995 in each arm) (Table 1). Compared to the control group, the vaccinated group had a significantly lower risk of pneumonia (RR 0.53 [95% CI 0.38-0.74]), ARF (RR 0.53 [95% CI 0.35-0.79]), hospital admissions (RR 0.50 [95% CI 0.25-0.99]), and ICU service utilization (RR 0.49 [95% CI 0.33-0.73]) (Figure 1). No cases of meningitis were reported in either group. For patients < 65 years old, compared to the unvaccinated subgroup, the vaccinated subgroup showed a lower risk of pneumonia (RR 0.37 [95% CI 0.21-0.65]), ARF (RR 0.40 [95% CI 0.19-0.82]), and ICU service utilization (RR 0.50 [95% CI 0.29-0.87]). Hospital admission rates were numerically lower but not significantly different in the vaccinated group (RR 0.59 [95% CI 0.27-1.27]) (Figure 1).
Discussion: This large database analysis revealed lower pneumonia risk and better health outcomes associated with pneumococcal vaccination in CD patients, strongly supporting CD's current pneumococcal vaccination guidelines. Further research is needed into the comparative effectiveness of different pneumococcal vaccine formulations.

Figure: Figure 1: Summary of outcomes of patients with celiac disease who received the pneumococcal vaccine compared to those who did not. A) All adults 18 years or older, and B) Patients aged 18-64 years.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mouhand Mohamed indicated no relevant financial relationships.
Osama Hamid indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Xiao Jing (Iris) Wang indicated no relevant financial relationships.
Adam Bledsoe: Chugai Pharma – Grant/Research Support. Kanyos Bio – Grant/Research Support.
Mouhand F.H. Mohamed, MD, MSc1, Osama Hamid, MD2, Azizullah Beran, MD3, Xiao Jing (Iris) Wang, MD1, Adam Bledsoe, MD1. P3200 - Pneumococcal Vaccine is Associated with Better Outcomes in Celiac Disease Patients: Insights From a Propensity-Matched Study in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
