Monday Poster Session
Category: Small Intestine
P3253 - A Case of Pembrolizumab-Induced Duodenitis Mimicking Celiac Disease
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Yannis Lafazanos, DO
Advocate Lutheran General
Park Ridge, IL
Presenting Author(s)
Yannis Lafazanos, DO1, Robin David, MD2, Nahren Asado, MBChB, MD1, Miguel Gonzalez, MD1, Eli D. Ehrenpreis, MD, FACG3
1Advocate Lutheran General, Park Ridge, IL; 2Advocate Lutheran General, Chicago, IL; 3E2Bio Consultants, Evanston, IL
Introduction: Pembrolizumab is a programmed death-1 (PD-1) inhibitor used in the treatment of various malignancies including lung cancer, melanoma, and Hodgkin lymphoma. While its immune upregulation proves efficacious against cancer cells, potentiation of immune activity predisposes patients to immune-related adverse effects (irAEs) such as pneumonitis, hepatitis, colitis and various endocrinopathies. Duodenitis is rare but represents significant clinical concern due to its severity and effect on quality of life. We report a case of pembrolizumab-induced enteropathy that endoscopically and histologically mimics celiac disease.
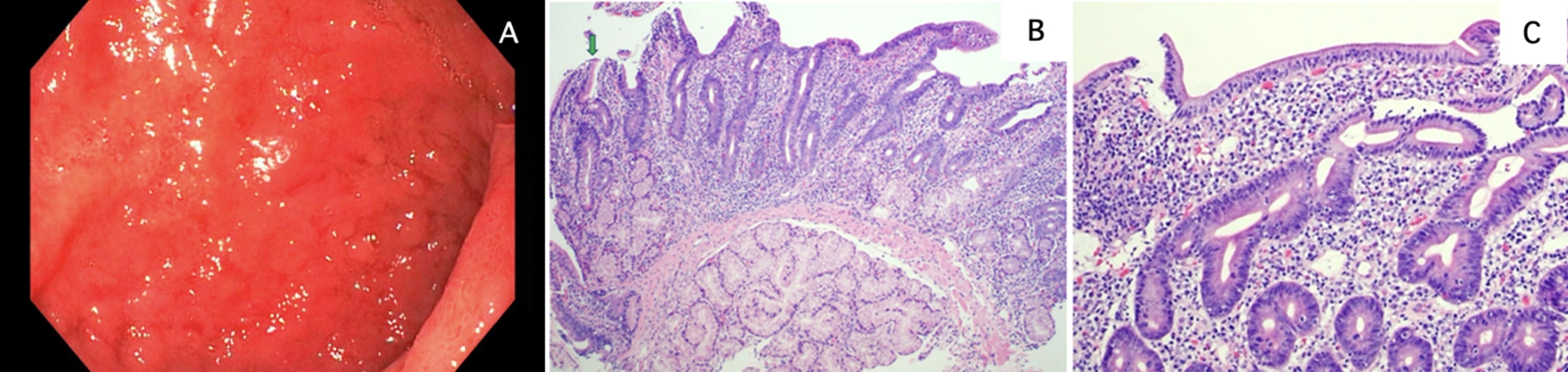
Case Description/Methods: A 64-year-old female with recently diagnosed stage IV lung adenocarcinoma status post two cycles of pembrolizumab presented to the ED with 3 weeks of profuse, watery diarrhea. She reported up to 15 episodes daily, not relieved by loperamide or trial of gluten-free diet. She denied recent antibiotic, laxative or NSAID use. She had no personal or family history of inflammatory bowel disease or celiac disease. Initial CBC and CMP were unremarkable and celiac serologies were negative. Infectious stool studies with C. Difficile and a pathogen panel including common viral, bacterial, and parasitic pathogens were unrevealing. Fecal calprotectin was normal. Imaging revealed moderate thickening of the duodenum. Endoscopic evaluation showed diffuse duodenal mucosal edema and nodularity with appearance similar to celiac disease (Image 1A). Biopsies showed acute on chronic inflammation, villous blunting (Image 1B), acute cryptitis, and intraepithelial lymphocytosis (Image 1C). Methylprednisolone 1 mg/kg daily was initiated for the presumptive diagnosis of immune-checkpoint inhibitor (ICI) duodenitis, and she dramatically improved. Her diarrhea resolved after completion of a prednisone taper. Pembrolizumab has been discontinued with plans for palliative radiation.
Discussion: Immunotherapies, including pembrolizumab, are commonplace in cancer treatment. While ICIs activate cytotoxic T-lymphocytes to target cancer cells, they can also cause irAEs. Upper gastrointestinal involvement is rare, with approximately 30 reported cases, and only 5 involving isolated duodenopathy. Dietary modifications with a gluten-free diet have been employed with varying degrees of success. The mainstay of treatment is immunosuppression, mainly through corticosteroids, which reduce inflammation and promote mucosal healing. Most cases resolve a week after discontinuing PD-1 inhibitors.
Disclosures:
Yannis Lafazanos, DO1, Robin David, MD2, Nahren Asado, MBChB, MD1, Miguel Gonzalez, MD1, Eli D. Ehrenpreis, MD, FACG3. P3253 - A Case of Pembrolizumab-Induced Duodenitis Mimicking Celiac Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Advocate Lutheran General, Park Ridge, IL; 2Advocate Lutheran General, Chicago, IL; 3E2Bio Consultants, Evanston, IL
Introduction: Pembrolizumab is a programmed death-1 (PD-1) inhibitor used in the treatment of various malignancies including lung cancer, melanoma, and Hodgkin lymphoma. While its immune upregulation proves efficacious against cancer cells, potentiation of immune activity predisposes patients to immune-related adverse effects (irAEs) such as pneumonitis, hepatitis, colitis and various endocrinopathies. Duodenitis is rare but represents significant clinical concern due to its severity and effect on quality of life. We report a case of pembrolizumab-induced enteropathy that endoscopically and histologically mimics celiac disease.
Case Description/Methods: A 64-year-old female with recently diagnosed stage IV lung adenocarcinoma status post two cycles of pembrolizumab presented to the ED with 3 weeks of profuse, watery diarrhea. She reported up to 15 episodes daily, not relieved by loperamide or trial of gluten-free diet. She denied recent antibiotic, laxative or NSAID use. She had no personal or family history of inflammatory bowel disease or celiac disease. Initial CBC and CMP were unremarkable and celiac serologies were negative. Infectious stool studies with C. Difficile and a pathogen panel including common viral, bacterial, and parasitic pathogens were unrevealing. Fecal calprotectin was normal. Imaging revealed moderate thickening of the duodenum. Endoscopic evaluation showed diffuse duodenal mucosal edema and nodularity with appearance similar to celiac disease (Image 1A). Biopsies showed acute on chronic inflammation, villous blunting (Image 1B), acute cryptitis, and intraepithelial lymphocytosis (Image 1C). Methylprednisolone 1 mg/kg daily was initiated for the presumptive diagnosis of immune-checkpoint inhibitor (ICI) duodenitis, and she dramatically improved. Her diarrhea resolved after completion of a prednisone taper. Pembrolizumab has been discontinued with plans for palliative radiation.
Discussion: Immunotherapies, including pembrolizumab, are commonplace in cancer treatment. While ICIs activate cytotoxic T-lymphocytes to target cancer cells, they can also cause irAEs. Upper gastrointestinal involvement is rare, with approximately 30 reported cases, and only 5 involving isolated duodenopathy. Dietary modifications with a gluten-free diet have been employed with varying degrees of success. The mainstay of treatment is immunosuppression, mainly through corticosteroids, which reduce inflammation and promote mucosal healing. Most cases resolve a week after discontinuing PD-1 inhibitors.

Figure: Image 1. Esophagogastroduodenoscopy of duodenum with pathology from biopsy. (A) Demonstration of diffuse intestinal mucosal edema and nodularity at the duodenal bulb. (B) Duodenal mucosa showing moderate villous blunting, and lamina propria expansion by acute and chronic inflammation, with focal foveolar mucin cell metaplasia (green arrow) (100x; H&E stain). (C) High power view of duodenal mucosa, showing mixed acute and chronic lamina propria inflammation with increased intraepithelial lymphocytes (200x; H&E stain).
Disclosures:
Yannis Lafazanos indicated no relevant financial relationships.
Robin David indicated no relevant financial relationships.
Nahren Asado indicated no relevant financial relationships.
Miguel Gonzalez indicated no relevant financial relationships.
Eli Ehrenpreis: E2Bio Life Sciences – Intellectual Property/Patents, Owner/Ownership Interest, Stock-privately held company.
Yannis Lafazanos, DO1, Robin David, MD2, Nahren Asado, MBChB, MD1, Miguel Gonzalez, MD1, Eli D. Ehrenpreis, MD, FACG3. P3253 - A Case of Pembrolizumab-Induced Duodenitis Mimicking Celiac Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

