Tuesday Poster Session
Category: Biliary/Pancreas
P3455 - Efficacy of Camostat Mesilate for Pain Related to Chronic Pancreatitis: A Systematic Review and Meta-Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
- AI
AbdiGhani Ismail, MD
Indiana University School of Medicine
Indianapolis, IN
Presenting Author(s)
AbdiGhani Ismail, MD, Saad Saadat, MD, Ahmad Karkash, MD, Jeffrey Easler, MD, Nasir Saleem, MD
Indiana University School of Medicine, Indianapolis, IN
Introduction: Chronic pancreatitis (CP) is characterized by inflammation that leads to progressive, irreversible tissue destruction and fibrosis of the pancreas. Pain is the most common symptom in CP. Camostat Mesilate (Camostat) is an oral serine protease inhibitor approved in Japan for treating painful CP. Through the inhibition of pancreatic proteases, Camostat is believed to inhibit inflammatory tissue damage and improve CP related pain symptoms.
The aim of this study is to evaluate the efficacy of Camostat in treating pain associated with CP. In the setting of limited English language studies exploring Camostat for this indication, our study is the first Meta-analysis on this topic
Methods: We conducted a comprehensive review of multiple databases including PubMed, Embase, Web of Science and SCOPUS. Studies were obtained based on pre-specified inclusion and exclusion criteria. Exclusion criteria included animal studies, lack of control group, non-CP intervention group, COVID-19 patients and non-English studies. Meta analysis was done using RevMan software. Statistical method used was Mantel-Haenszel. Analysis model used was random effects regardless of heterogeneity.
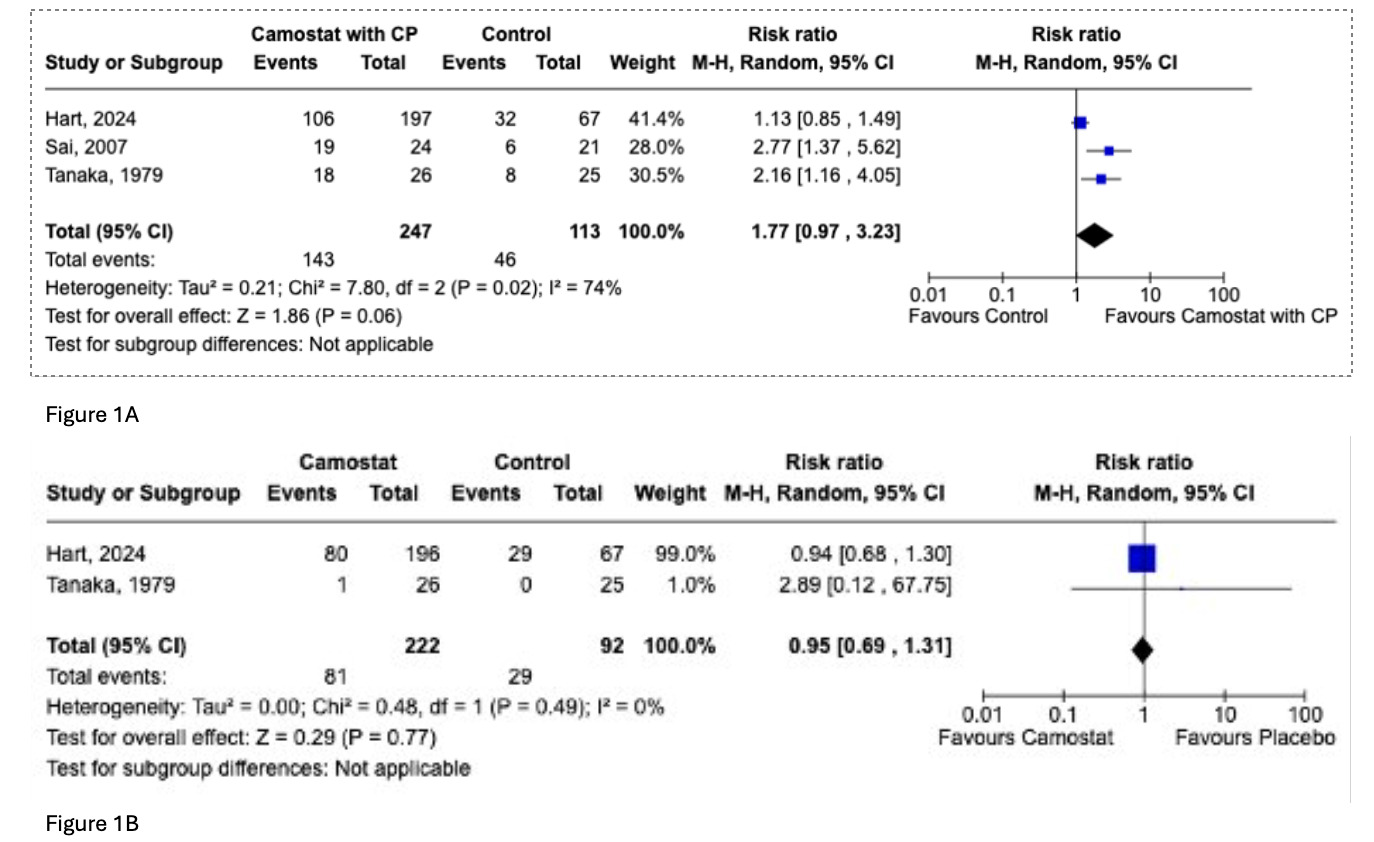
Results: Three studies (2 double-blind Randomized controlled trials and 1 single center prospective study) encompassing 360 patients (49.1% female) met study criteria. Primary outcome measured was pain relief as defined by criteria outlined by the individual studies included in this analysis. Between the 3 studies no significant difference in pain relief was seen between subjects that received Camostat intervention versus controls (RR 1.77, 95% CI [0.97 , 3.23], P = 0.06, I² = 74% (Figure 1a). There were no significant difference in overall adverse events between Camostat and placebo ( RR 0.95, 95% CI (0.69 , 1.31], P = 0.77), I² = 0% (Figure 1b)
Discussion: The results of our meta-analysis suggest patients with painful CP have no significant improvement in pain with Camostat intervention. However, firm conclusions on the efficacy of Camostat remain limited in the setting of few rigorous, prospective studies and significant heterogeneity in methods across studies. More large scale RCTs are needed to evaluate the efficacy of Camostat for painful CP.
Disclosures:
AbdiGhani Ismail, MD, Saad Saadat, MD, Ahmad Karkash, MD, Jeffrey Easler, MD, Nasir Saleem, MD. P3455 - Efficacy of Camostat Mesilate for Pain Related to Chronic Pancreatitis: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Indiana University School of Medicine, Indianapolis, IN
Introduction: Chronic pancreatitis (CP) is characterized by inflammation that leads to progressive, irreversible tissue destruction and fibrosis of the pancreas. Pain is the most common symptom in CP. Camostat Mesilate (Camostat) is an oral serine protease inhibitor approved in Japan for treating painful CP. Through the inhibition of pancreatic proteases, Camostat is believed to inhibit inflammatory tissue damage and improve CP related pain symptoms.
The aim of this study is to evaluate the efficacy of Camostat in treating pain associated with CP. In the setting of limited English language studies exploring Camostat for this indication, our study is the first Meta-analysis on this topic
Methods: We conducted a comprehensive review of multiple databases including PubMed, Embase, Web of Science and SCOPUS. Studies were obtained based on pre-specified inclusion and exclusion criteria. Exclusion criteria included animal studies, lack of control group, non-CP intervention group, COVID-19 patients and non-English studies. Meta analysis was done using RevMan software. Statistical method used was Mantel-Haenszel. Analysis model used was random effects regardless of heterogeneity.
Results: Three studies (2 double-blind Randomized controlled trials and 1 single center prospective study) encompassing 360 patients (49.1% female) met study criteria. Primary outcome measured was pain relief as defined by criteria outlined by the individual studies included in this analysis. Between the 3 studies no significant difference in pain relief was seen between subjects that received Camostat intervention versus controls (RR 1.77, 95% CI [0.97 , 3.23], P = 0.06, I² = 74% (Figure 1a). There were no significant difference in overall adverse events between Camostat and placebo ( RR 0.95, 95% CI (0.69 , 1.31], P = 0.77), I² = 0% (Figure 1b)
Discussion: The results of our meta-analysis suggest patients with painful CP have no significant improvement in pain with Camostat intervention. However, firm conclusions on the efficacy of Camostat remain limited in the setting of few rigorous, prospective studies and significant heterogeneity in methods across studies. More large scale RCTs are needed to evaluate the efficacy of Camostat for painful CP.

Figure: Figure 1a. Pain relief in CP in camostat intervention group vs control as determined by study
Figure 1b. Treatment related adverse effects between two randomized controlled trials, patients received either camostat or placebo
Figure 1b. Treatment related adverse effects between two randomized controlled trials, patients received either camostat or placebo
Disclosures:
AbdiGhani Ismail indicated no relevant financial relationships.
Saad Saadat indicated no relevant financial relationships.
Ahmad Karkash indicated no relevant financial relationships.
Jeffrey Easler: Boston Scientific – Consultant.
Nasir Saleem indicated no relevant financial relationships.
AbdiGhani Ismail, MD, Saad Saadat, MD, Ahmad Karkash, MD, Jeffrey Easler, MD, Nasir Saleem, MD. P3455 - Efficacy of Camostat Mesilate for Pain Related to Chronic Pancreatitis: A Systematic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
