Tuesday Poster Session
Category: Biliary/Pancreas
P3552 - Metastatic Pancreatic Adenocarcinoma: A Rare Case of Successful Checkpoint Inhibitor Monotherapy in High Tumor Mutational Burden With POLE Gene
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Karthic Drishna Perumal, MD
Mount Carmel Health System, OH
Presenting Author(s)
Karthic Drishna Perumal, MD1, Kenneth Chian, 2, Mingjia Li, MD3, Arjun Mittra, MD3
1Mount Carmel Health System, Powell, OH; 2The Ohio State University College of Medicine, Columbus, OH; 3The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Pancreatic ductal adenocarcinoma is a malignancy originating from the pancreatic duct cells. Many patients are diagnosed in a latent stage III or stage IV disease and are often refractory to chemotherapy or surgical treatment. Checkpoint inhibitor therapy can activate and mobilize host T-cells to attack cancer cells. Furthermore, it has been studied that a higher tumor mutation burden in solid tumors has proven fruitful towards higher response rates with checkpoint inhibitor therapy as well.
Case Description/Methods: We present a 73 year old male with a past medical and family history significant for squamous cell skin cancer and several first degree relatives with lung cancer, non-Hodgkin’s Lymphoma, and chronic lymphocytic leukemia, who presented to the oncology clinic with 3 months of abdominal pain, bloating, and weight loss. Abdominal computed tomography (CT) showed a hypodense mass in the proximal body of the pancreas with encasement of the celiac and common hepatic artery. A subsequent abdominal magnetic resonance imaging revealed numerous metastatic lesions in the liver and kidney. Pancreatic biopsy confirmed adenocarcinoma with a CA 19-9 of 2663. He received four cycles of Folfirinox showing initial improvement in reducing vascular encasement and received several more cycles of Gemcitabine/Abraxane/Cisplatin after next generation sequencing revealed BRCA mutations. The patient was then enrolled in a clinical trial that sent a liver biopsy for molecular testing, revealing a very high tumor mutation burden (TMB), but no microsatellite instability, suggestive of a POLE mutation. He started Pembrolizumab 200 mg monotherapy on a 21-day cycle and continues to exhibit excellent response with successive CT scans revealing a decrease in size of multiple hepatic lesions, pancreatic mass, and CA 19-9 to 188.23.
Discussion: Pembrolizumab is a checkpoint inhibitor promoting immune activation amongst cells with the programmed death checkpoint inhibitor (PD-1) receptor, which are otherwise evaded by tumors displaying programmed death protein ligands 1 & 2 ligands on their surface. Monotherapy often proves unsuccessful, but our patient benefitted from monotherapy due to unique POLE mutation and high TMB, which has been studied to increase successful response to checkpoint inhibitor therapy. However, there still remains many technological restraints, variation across tumor types, thresholds for therapeutic PD-1 expression, and side effects necessitating further future clinical studies and trials.
Disclosures:
Karthic Drishna Perumal, MD1, Kenneth Chian, 2, Mingjia Li, MD3, Arjun Mittra, MD3. P3552 - Metastatic Pancreatic Adenocarcinoma: A Rare Case of Successful Checkpoint Inhibitor Monotherapy in High Tumor Mutational Burden With POLE Gene, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mount Carmel Health System, Powell, OH; 2The Ohio State University College of Medicine, Columbus, OH; 3The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Pancreatic ductal adenocarcinoma is a malignancy originating from the pancreatic duct cells. Many patients are diagnosed in a latent stage III or stage IV disease and are often refractory to chemotherapy or surgical treatment. Checkpoint inhibitor therapy can activate and mobilize host T-cells to attack cancer cells. Furthermore, it has been studied that a higher tumor mutation burden in solid tumors has proven fruitful towards higher response rates with checkpoint inhibitor therapy as well.
Case Description/Methods: We present a 73 year old male with a past medical and family history significant for squamous cell skin cancer and several first degree relatives with lung cancer, non-Hodgkin’s Lymphoma, and chronic lymphocytic leukemia, who presented to the oncology clinic with 3 months of abdominal pain, bloating, and weight loss. Abdominal computed tomography (CT) showed a hypodense mass in the proximal body of the pancreas with encasement of the celiac and common hepatic artery. A subsequent abdominal magnetic resonance imaging revealed numerous metastatic lesions in the liver and kidney. Pancreatic biopsy confirmed adenocarcinoma with a CA 19-9 of 2663. He received four cycles of Folfirinox showing initial improvement in reducing vascular encasement and received several more cycles of Gemcitabine/Abraxane/Cisplatin after next generation sequencing revealed BRCA mutations. The patient was then enrolled in a clinical trial that sent a liver biopsy for molecular testing, revealing a very high tumor mutation burden (TMB), but no microsatellite instability, suggestive of a POLE mutation. He started Pembrolizumab 200 mg monotherapy on a 21-day cycle and continues to exhibit excellent response with successive CT scans revealing a decrease in size of multiple hepatic lesions, pancreatic mass, and CA 19-9 to 188.23.
Discussion: Pembrolizumab is a checkpoint inhibitor promoting immune activation amongst cells with the programmed death checkpoint inhibitor (PD-1) receptor, which are otherwise evaded by tumors displaying programmed death protein ligands 1 & 2 ligands on their surface. Monotherapy often proves unsuccessful, but our patient benefitted from monotherapy due to unique POLE mutation and high TMB, which has been studied to increase successful response to checkpoint inhibitor therapy. However, there still remains many technological restraints, variation across tumor types, thresholds for therapeutic PD-1 expression, and side effects necessitating further future clinical studies and trials.

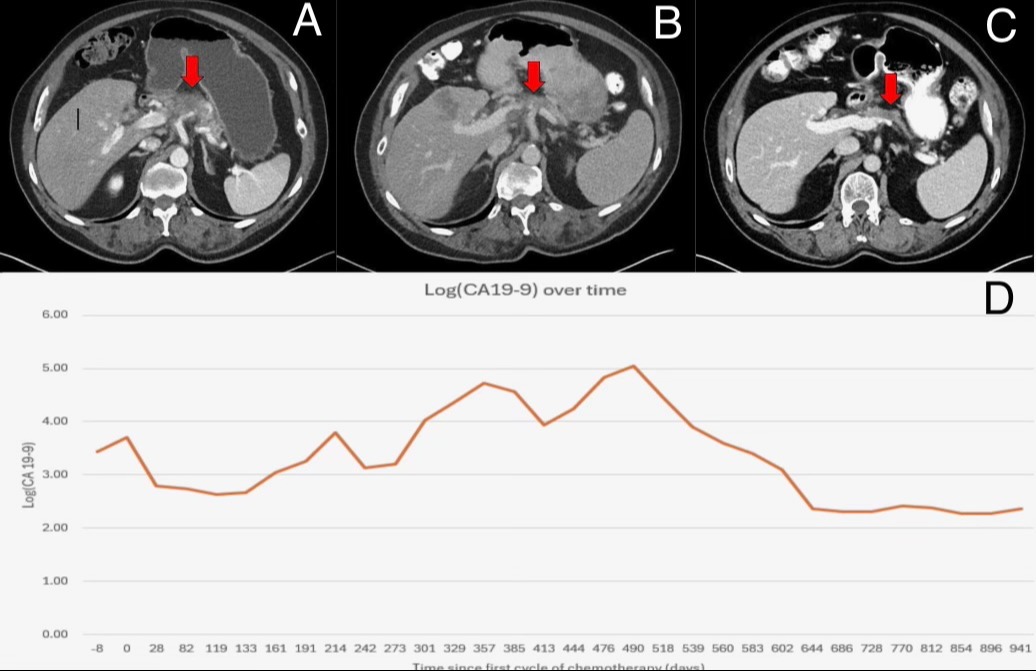
Figure: A) CT scan before initiating chemotherapy showing pancreatic mass with involvement of the celiac and common hepatic artery
B) CT scan before initiating immunotherapy (day 497 of chemotherapy) showing pancreatic mass with similar vascular involvement and now 2 new metastatic hepatic lesions
C) CT scan 434 days after immunotherapy initiation with decrease in size of pancreatic mass and hepatic lesions
D) Logarithmic graph of CA 19-9 levels over time since initiating chemotherapy and subsequent immunotherapy
B) CT scan before initiating immunotherapy (day 497 of chemotherapy) showing pancreatic mass with similar vascular involvement and now 2 new metastatic hepatic lesions
C) CT scan 434 days after immunotherapy initiation with decrease in size of pancreatic mass and hepatic lesions
D) Logarithmic graph of CA 19-9 levels over time since initiating chemotherapy and subsequent immunotherapy
Disclosures:
Karthic Drishna Perumal indicated no relevant financial relationships.
Kenneth Chian indicated no relevant financial relationships.
Mingjia Li indicated no relevant financial relationships.
Arjun Mittra indicated no relevant financial relationships.
Karthic Drishna Perumal, MD1, Kenneth Chian, 2, Mingjia Li, MD3, Arjun Mittra, MD3. P3552 - Metastatic Pancreatic Adenocarcinoma: A Rare Case of Successful Checkpoint Inhibitor Monotherapy in High Tumor Mutational Burden With POLE Gene, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
