Tuesday Poster Session
Category: Colon
P3653 - Randomized Clinical Trial of Infliximab Versus Vedolizumab for Immune Checkpoint Inhibitor Related Colitis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- CC
Carolina Colli Cruz, MD
MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Award: Presidential Poster Award
Yinghong Wang, MD, PhD1, Krishnavathana Varatharajalu, MD2, Malek Shatila, MD2, Carolina Colli Cruz, MD3, Kristin Junek, ARNP2, Ninoska Silva, NP2, Anusha Thomas, MD2
1University of Texas MD Anderson Cancer Center, Houston, TX; 2The University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX
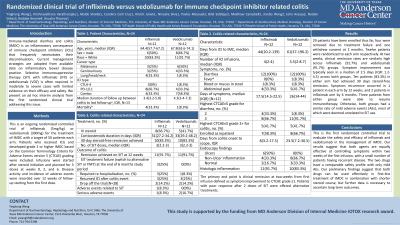
Introduction: Immune-mediated diarrhea and colitis (IMDC) is an inflammatory consequence of immunotherapy. Current management strategies are adopted from inflammatory bowel disease practice and rely on selective immunosuppressive therapy (SIT) with infliximab or vedolizumab in more severe cases. However, evidence supporting SIT use is limited to retrospective studies. Here we present the preliminary findings of 28 patients from the first clinical trial comparing the efficacy and safety of infliximab and vedolizumab in treating IMDC.
Methods: We conducted a randomized controlled trial of infliximab and vedolizumab in the treatment of IMDC in combination with steroid treatment. Patients included must have received immunotherapy and developed IMDC at CTCAE grade ≥2. Infusions were given around weeks 0, 2, and 6, and disease activity and adverse events were recorded over 12 weeks. Response and remission was reported at different timepoints. Fecal transplantation or ustekinumab would be considered for refractory cases after two SIT doses.
Results: 29 patients have been enrolled thus far, four were removed due to treatment failure and one withdrew consent at 2 months. Twelve patients were randomized to each arm respectively. At two weeks, clinical remission rates are similarly high across infliximab (91.7%) and vedolizumab (91.7%) groups. Symptom improvement was typically seen in a median of 2.5 days (IQR: 1.2-4.5) across both groups. Ten patients (83.3%) in either arm had achieved 30 days steroid-free remission. Symptom recurrence occurred in 1 patient in each arm by 12 weeks, and 2 patients in infliximab arm by 6 months. Three patients from either group were able to resume immunotherapy. Otherwise, both groups had a similar rate of mild adverse events (AEs), most of which were deemed unrelated to SIT use.
Discussion: This is the first randomized controlled trial to evaluate the safety and efficacy of infliximab and vedolizumab in the management of IMDC. Our results suggest that both agents are equally effective at controlling symptoms within two weeks of the first infusion, with a small number of patients having recurrent disease. The two drugs have a comparable safety profile with only mild AEs. Our preliminary findings suggest that both drugs can be used effectively in first-line treatment of IMDC in combination with shorter steroid course, but further data is necessary to ascertain long-term outcomes.
Disclosures:
Yinghong Wang, MD, PhD1, Krishnavathana Varatharajalu, MD2, Malek Shatila, MD2, Carolina Colli Cruz, MD3, Kristin Junek, ARNP2, Ninoska Silva, NP2, Anusha Thomas, MD2. P3653 - Randomized Clinical Trial of Infliximab Versus Vedolizumab for Immune Checkpoint Inhibitor Related Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Yinghong Wang, MD, PhD1, Krishnavathana Varatharajalu, MD2, Malek Shatila, MD2, Carolina Colli Cruz, MD3, Kristin Junek, ARNP2, Ninoska Silva, NP2, Anusha Thomas, MD2
1University of Texas MD Anderson Cancer Center, Houston, TX; 2The University of Texas MD Anderson Cancer Center, Houston, TX; 3MD Anderson Cancer Center, Houston, TX
Introduction: Immune-mediated diarrhea and colitis (IMDC) is an inflammatory consequence of immunotherapy. Current management strategies are adopted from inflammatory bowel disease practice and rely on selective immunosuppressive therapy (SIT) with infliximab or vedolizumab in more severe cases. However, evidence supporting SIT use is limited to retrospective studies. Here we present the preliminary findings of 28 patients from the first clinical trial comparing the efficacy and safety of infliximab and vedolizumab in treating IMDC.
Methods: We conducted a randomized controlled trial of infliximab and vedolizumab in the treatment of IMDC in combination with steroid treatment. Patients included must have received immunotherapy and developed IMDC at CTCAE grade ≥2. Infusions were given around weeks 0, 2, and 6, and disease activity and adverse events were recorded over 12 weeks. Response and remission was reported at different timepoints. Fecal transplantation or ustekinumab would be considered for refractory cases after two SIT doses.
Results: 29 patients have been enrolled thus far, four were removed due to treatment failure and one withdrew consent at 2 months. Twelve patients were randomized to each arm respectively. At two weeks, clinical remission rates are similarly high across infliximab (91.7%) and vedolizumab (91.7%) groups. Symptom improvement was typically seen in a median of 2.5 days (IQR: 1.2-4.5) across both groups. Ten patients (83.3%) in either arm had achieved 30 days steroid-free remission. Symptom recurrence occurred in 1 patient in each arm by 12 weeks, and 2 patients in infliximab arm by 6 months. Three patients from either group were able to resume immunotherapy. Otherwise, both groups had a similar rate of mild adverse events (AEs), most of which were deemed unrelated to SIT use.
Discussion: This is the first randomized controlled trial to evaluate the safety and efficacy of infliximab and vedolizumab in the management of IMDC. Our results suggest that both agents are equally effective at controlling symptoms within two weeks of the first infusion, with a small number of patients having recurrent disease. The two drugs have a comparable safety profile with only mild AEs. Our preliminary findings suggest that both drugs can be used effectively in first-line treatment of IMDC in combination with shorter steroid course, but further data is necessary to ascertain long-term outcomes.
Disclosures:
Yinghong Wang: AzurRx – Consultant. Ilyapharma – Consultant. IOTA – Consultant. Sorriso – Consultant. Tillotts – Consultant.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Malek Shatila indicated no relevant financial relationships.
Carolina Colli Cruz indicated no relevant financial relationships.
Kristin Junek indicated no relevant financial relationships.
Ninoska Silva indicated no relevant financial relationships.
Anusha Thomas indicated no relevant financial relationships.
Yinghong Wang, MD, PhD1, Krishnavathana Varatharajalu, MD2, Malek Shatila, MD2, Carolina Colli Cruz, MD3, Kristin Junek, ARNP2, Ninoska Silva, NP2, Anusha Thomas, MD2. P3653 - Randomized Clinical Trial of Infliximab Versus Vedolizumab for Immune Checkpoint Inhibitor Related Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

