Tuesday Poster Session
Category: Colon
P3691 - Refractory Immune Checkpoint Inhibitor-Associated Colitis: Case Report
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E


Nurlan Aliyev, MD
University of Nebraska Medical Center
Omaha, NE
Presenting Author(s)
Nurlan Aliyev, MD, Meagan S. Reif, MD, Natapat Chaisidhivej, MD, Pauline Yasmeh, MD, Jacob C. Middleton, MD, Romana A. Bhat, MD, Suha J. Jabak, MD
University of Nebraska Medical Center, Omaha, NE
Introduction: Ipilimumab and Nivolumab are immune checkpoint inhibitors (ICIs) gaining popularity as anticancer agents that improve survival. Ipilimumab blocks the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), while Nivolumab blocks the programmed death 1 receptor (PD-1) to inhibit immune checkpoints. The combination of Ipilimumab and Nivolumab (Ipi/Nivo) is approved for metastatic non-small cell lung cancer, renal cell carcinoma, and melanoma. ICIs have many potential immune-mediated side effects involving the gut, from mild diarrhea to life-threatening colitis.
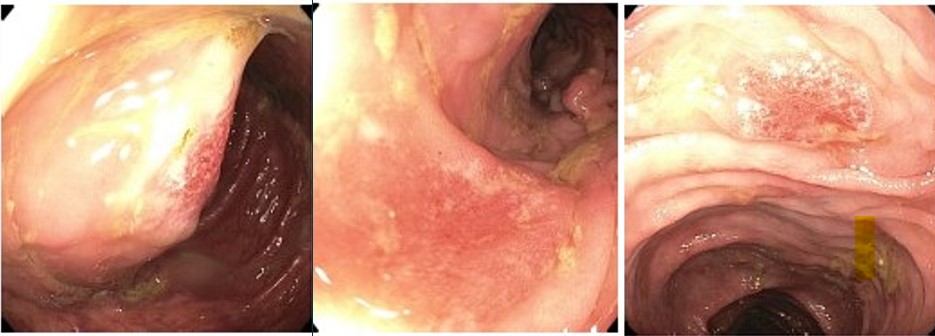
Case Description/Methods: A 61-year-old male with metastatic melanoma presented with four days of non-bloody diarrhea with >10 stools ( >2L) per day. He endorsed poor appetite, fatigue, nausea, and abdominal cramping without fever or hematochezia. He received last Ipi/Nivo infusion two weeks before symptom onset. The patient had normal vitals, and non-distended, non-tender abdomen. Labs showed mild leukocytosis to 12,200/mm3 and hematocrit 46%. Stool and blood cultures were negative. Colonoscopy showed discontinuous, non-bleeding mucosal ulcerations throughout the colon, notably worse on the right. Random biopsies revealed inflammation in the colonic lamina propria, erosions, and ulcerations with apoptotic bodies, negative Cytomegalovirus, consistent with ICI colitis. The patient started 1 mg/kg/day of oral Prednisone. His diarrhea persisted despite dose escalation to 1.5 mg/kg/day IV Methylprednisolone for several days. He was then given a 5 mg/kg Infliximab infusion with marked improvement within two days to 100mL of daily stool from the prior 2L. He was transitioned to oral steroids with plan to start slow taper after two weeks. ICI therapy were discontinued.
Discussion: ICI colitis usually develops 6-8 weeks after ICI infusion and commonly presents with diarrhea and abdominal pain. It is associated with increased mortality, especially with combined ICIs (e.g., Ipi/Nivo) as compared to single agents. Early diagnosis and treatment are critical as patients may quickly develop ileus, toxic megacolon, bowel perforation, and death. ICI colitis with stool frequency of 7 or more over baseline per day is considered severe using Common Terminology Criteria for Adverse Events and classified as grades 3-5. These patients require prompt hospitalization for high-dose IV steroids at 1-2 mg/kg/day, and biologic treatment in steroid-refractory cases, and the implicated ICI(s) should be permanently discontinued if diagnosed with grade 3 or 4 colitis.
Disclosures:
Nurlan Aliyev, MD, Meagan S. Reif, MD, Natapat Chaisidhivej, MD, Pauline Yasmeh, MD, Jacob C. Middleton, MD, Romana A. Bhat, MD, Suha J. Jabak, MD. P3691 - Refractory Immune Checkpoint Inhibitor-Associated Colitis: Case Report, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
University of Nebraska Medical Center, Omaha, NE
Introduction: Ipilimumab and Nivolumab are immune checkpoint inhibitors (ICIs) gaining popularity as anticancer agents that improve survival. Ipilimumab blocks the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), while Nivolumab blocks the programmed death 1 receptor (PD-1) to inhibit immune checkpoints. The combination of Ipilimumab and Nivolumab (Ipi/Nivo) is approved for metastatic non-small cell lung cancer, renal cell carcinoma, and melanoma. ICIs have many potential immune-mediated side effects involving the gut, from mild diarrhea to life-threatening colitis.
Case Description/Methods: A 61-year-old male with metastatic melanoma presented with four days of non-bloody diarrhea with >10 stools ( >2L) per day. He endorsed poor appetite, fatigue, nausea, and abdominal cramping without fever or hematochezia. He received last Ipi/Nivo infusion two weeks before symptom onset. The patient had normal vitals, and non-distended, non-tender abdomen. Labs showed mild leukocytosis to 12,200/mm3 and hematocrit 46%. Stool and blood cultures were negative. Colonoscopy showed discontinuous, non-bleeding mucosal ulcerations throughout the colon, notably worse on the right. Random biopsies revealed inflammation in the colonic lamina propria, erosions, and ulcerations with apoptotic bodies, negative Cytomegalovirus, consistent with ICI colitis. The patient started 1 mg/kg/day of oral Prednisone. His diarrhea persisted despite dose escalation to 1.5 mg/kg/day IV Methylprednisolone for several days. He was then given a 5 mg/kg Infliximab infusion with marked improvement within two days to 100mL of daily stool from the prior 2L. He was transitioned to oral steroids with plan to start slow taper after two weeks. ICI therapy were discontinued.
Discussion: ICI colitis usually develops 6-8 weeks after ICI infusion and commonly presents with diarrhea and abdominal pain. It is associated with increased mortality, especially with combined ICIs (e.g., Ipi/Nivo) as compared to single agents. Early diagnosis and treatment are critical as patients may quickly develop ileus, toxic megacolon, bowel perforation, and death. ICI colitis with stool frequency of 7 or more over baseline per day is considered severe using Common Terminology Criteria for Adverse Events and classified as grades 3-5. These patients require prompt hospitalization for high-dose IV steroids at 1-2 mg/kg/day, and biologic treatment in steroid-refractory cases, and the implicated ICI(s) should be permanently discontinued if diagnosed with grade 3 or 4 colitis.

Figure: Mucosal ulcerations of colon
Disclosures:
Nurlan Aliyev indicated no relevant financial relationships.
Meagan Reif indicated no relevant financial relationships.
Natapat Chaisidhivej indicated no relevant financial relationships.
Pauline Yasmeh indicated no relevant financial relationships.
Jacob Middleton indicated no relevant financial relationships.
Romana Bhat indicated no relevant financial relationships.
Suha Jabak indicated no relevant financial relationships.
Nurlan Aliyev, MD, Meagan S. Reif, MD, Natapat Chaisidhivej, MD, Pauline Yasmeh, MD, Jacob C. Middleton, MD, Romana A. Bhat, MD, Suha J. Jabak, MD. P3691 - Refractory Immune Checkpoint Inhibitor-Associated Colitis: Case Report, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
