Tuesday Poster Session
Category: Colorectal Cancer Prevention
P3854 - Glucagon-Like Peptide-1 Receptor Agonist Use and its Potential Impact in Colonoscopy Among Patients Undergoing Colorectal Cancer Screening: A Multicenter Propensity Score Matching Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Ahmed Eltelbany, MD, MPH
University of New Mexico
Albuquerque, NM
Presenting Author(s)
Ahmed El Telbany, MD, MPH1, Mounir Ibrahim, MD2, Islam Mohamed, MD3, Swathi Paleti, MD1
1University of New Mexico, Albuquerque, NM; 2Cleveland Clinic Foundation, Holmdel, NJ; 3University of Missouri - Kansas City School of Medicine, Kansas City, MO
Introduction: Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are increasingly used for the treatment of type 2 diabetes mellitus and obesity. These medications slow gastric emptying and increase colonic transit time, which may impact the quality of bowel preparation and completeness of colonoscopy. An incomplete colonoscopy can lead to missed colorectal neoplasia and the need for repeat colonoscopy. To date, the association between GLP1-RA use and incomplete colonoscopy has not been well established. In our study, we aim to investigate the association between GLP1-RA use and the incidence of repeat colonoscopy within one year.
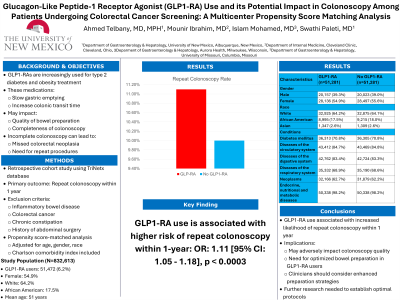
Methods: We conducted a retrospective cohort study using a large, nationally representative database, TriNetx to evaluate the association between GLP1-RA use and repeat colonoscopy in patients undergoing colorectal cancer screening. Patients were categorized as GLP1-RA users or non-users based on medication records. The primary outcome was defined as a repeat colonoscopy within 1 year. Patients with a history of inflammatory bowel disease, colorectal cancer, chronic constipation, and a history of abdominal surgery were excluded from the study. Patient demographics, co-morbidities, medications, and colonoscopy outcomes were extracted. Propensity score-matched Cox regression analysis (Table 1) was performed to adjust for potential confounders including age, gender, race and Charlson comorbidity index. E-values were calculated to assess the robustness of the findings.
Results: The analysis included 832,613 patients who underwent colonoscopy, of which 51,472 (6.2%) were GLP1-RA users. Among these patients 54.9% were females, 64.2% were white and 17.5% were African American with a mean age of 51 years (Table 1). A propensity-score matched analysis revealed that GLP1-RA use was associated with a higher risk of repeat colonoscopy within 1-year odds ratio (OR) 1.11; [95% CI 1.05 - 1.18, p< 0.0003] compared to non-GLP1-RA use.
Discussion: In a large, propensity score-matched cohort, GLP1-RA use was associated with an increased likelihood of repeat colonoscopy within 1 year in patients undergoing colorectal cancer screening. These findings suggest that GLP1-RA use may adversely impact the quality of colonoscopy and highlight the need for further research to optimize bowel preparation and colonoscopy outcomes in this population. Clinicians should be aware of this potential risk when prescribing GLP1-RAs and consider enhanced bowel preparation strategies in these patients.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ahmed El Telbany, MD, MPH1, Mounir Ibrahim, MD2, Islam Mohamed, MD3, Swathi Paleti, MD1. P3854 - Glucagon-Like Peptide-1 Receptor Agonist Use and its Potential Impact in Colonoscopy Among Patients Undergoing Colorectal Cancer Screening: A Multicenter Propensity Score Matching Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of New Mexico, Albuquerque, NM; 2Cleveland Clinic Foundation, Holmdel, NJ; 3University of Missouri - Kansas City School of Medicine, Kansas City, MO
Introduction: Glucagon-like peptide-1 receptor agonists (GLP1-RAs) are increasingly used for the treatment of type 2 diabetes mellitus and obesity. These medications slow gastric emptying and increase colonic transit time, which may impact the quality of bowel preparation and completeness of colonoscopy. An incomplete colonoscopy can lead to missed colorectal neoplasia and the need for repeat colonoscopy. To date, the association between GLP1-RA use and incomplete colonoscopy has not been well established. In our study, we aim to investigate the association between GLP1-RA use and the incidence of repeat colonoscopy within one year.
Methods: We conducted a retrospective cohort study using a large, nationally representative database, TriNetx to evaluate the association between GLP1-RA use and repeat colonoscopy in patients undergoing colorectal cancer screening. Patients were categorized as GLP1-RA users or non-users based on medication records. The primary outcome was defined as a repeat colonoscopy within 1 year. Patients with a history of inflammatory bowel disease, colorectal cancer, chronic constipation, and a history of abdominal surgery were excluded from the study. Patient demographics, co-morbidities, medications, and colonoscopy outcomes were extracted. Propensity score-matched Cox regression analysis (Table 1) was performed to adjust for potential confounders including age, gender, race and Charlson comorbidity index. E-values were calculated to assess the robustness of the findings.
Results: The analysis included 832,613 patients who underwent colonoscopy, of which 51,472 (6.2%) were GLP1-RA users. Among these patients 54.9% were females, 64.2% were white and 17.5% were African American with a mean age of 51 years (Table 1). A propensity-score matched analysis revealed that GLP1-RA use was associated with a higher risk of repeat colonoscopy within 1-year odds ratio (OR) 1.11; [95% CI 1.05 - 1.18, p< 0.0003] compared to non-GLP1-RA use.
Discussion: In a large, propensity score-matched cohort, GLP1-RA use was associated with an increased likelihood of repeat colonoscopy within 1 year in patients undergoing colorectal cancer screening. These findings suggest that GLP1-RA use may adversely impact the quality of colonoscopy and highlight the need for further research to optimize bowel preparation and colonoscopy outcomes in this population. Clinicians should be aware of this potential risk when prescribing GLP1-RAs and consider enhanced bowel preparation strategies in these patients.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ahmed El Telbany indicated no relevant financial relationships.
Mounir Ibrahim indicated no relevant financial relationships.
Islam Mohamed indicated no relevant financial relationships.
Swathi Paleti indicated no relevant financial relationships.
Ahmed El Telbany, MD, MPH1, Mounir Ibrahim, MD2, Islam Mohamed, MD3, Swathi Paleti, MD1. P3854 - Glucagon-Like Peptide-1 Receptor Agonist Use and its Potential Impact in Colonoscopy Among Patients Undergoing Colorectal Cancer Screening: A Multicenter Propensity Score Matching Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
